Medicaid Prescription Drugs Covered Outpatient Drug Final Rule













- Slides: 26

Medicaid Prescription Drugs Covered Outpatient Drug Final Rule with Comment: Implementing the Provisions of the Affordable Care Act For information, proposals and specific details regarding the notice of proposed rulemaking (NPRM) discussed in this slide deck, please see the NPRM entitled: “Medicaid; Covered Outpatient Drugs” that was published in the Federal Register on February 2, 2012 (77 FR 5318). This slide deck is not intended to replace or be considered in lieu of the NPRM or final rule with comment. In addition, these slides do not represent viewpoints or interpretations by the Centers for Medicare & Medicaid Services (CMS) that are in addition or contrary to those articulated in the NPRM or final rule. Because the information in this slide deck is only intended to be a general summary and is not a complete or full summary of the NPRM or final rule with comment, readers should consult and review the NPRM and final rule with comment for a full and accurate statement of its contents. 1

Covered Outpatient Drug Rule CMS-2345 -FC • NPRM published February 2, 2012. • Final rule with comment published February 1, 2016. • Over 425 commenters submitted comments. • Effective date is April 1, 2016. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 2

Tribal Consultation • Valuable in helping CMS to finalize policies and support Indian health programs. We obtained the advice and input of Tribal officials on – February 23, 2012 during the Tribal Technical Advisory Group (TTAG) face-to-face meeting in Washington, DC , and – March 16, 2012 during an All Tribes’ Call under Executive Order 13175 and the CMS HHS Tribal Consultation Policy (November 2011). – Throughout the regulatory review process. • In determining final policies and regulations, all comments received before the close of the comment period including those received through Tribal consultations were considered. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 3

Key Provisions of the Final Rule with Comment The key provisions of this final rule with comment (final rule) fall under one of three categories: (1) Pharmacy Reimbursement; (2) Manufacturer Reporting Requirements; and (3) Rebate Requirements. We will be discussing areas in (1) and (2) that apply to IHS, Tribal, and Urban Indian Organizations (I/T/U) as well as facilities that serve American Indians and Alaska Natives. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 4

Pharmacy Reimbursement In this section we will cover: • Actual Acquisition Cost (AAC) • Professional Dispensing Fee • Reimbursement for Federal Discount Programs (340 B and Federal Supply Schedule (FSS)) • Federal Upper Limits (FUL) This information is only intended to be a general summary and is not a complete or full summary of the final rule. 5

Actual Acquisition Cost (AAC) Final Rule: • Defines AAC (§ 447. 502) to mean the agency’s determination of the pharmacy providers’ actual prices paid to acquire drug products marketed or sold by specific manufacturers. • Replaces estimated acquisition cost (EAC) with AAC in § 447. 512(b). • Explains that the change to AAC was necessary as it represents a more accurate reference price to be used by states to reimburse providers for drugs. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 6

AAC • In accordance with the requirements of § 447. 512(b) payment for brand name drugs and for drugs for which a FULs is not established, payment must not exceed, in the aggregate, the lower of: § AAC plus a professional dispensing fee; or § Provider’s usual and customary charges to the general public. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 7

Professional Dispensing Fee Final Rule: • Finalizes replacing “dispensing fee” with “professional dispensing fee” in § 447. 502 (as applied in § 447. 512(b)). • Reinforces CMS’ position that the fee to dispense the drug to a Medicaid beneficiary should reflect the pharmacist’s professional services and costs. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 8

Professional Dispensing Fee • States have the flexibility to set their professional dispensing fee. • State can use, but are not limited to, one of the following methods to establish their professional dispensing fee: § National survey/data § Regional/neighboring state survey/data § State-specific survey/data This information is only intended to be a general summary and is not a complete or full summary of the final rule. 9

State Plan Requirements • States have four quarters from the effective date of the final rule, which is April 1, 2016, to revise their state plan and submit a SPA with an effective date no later than April 1, 2017 to comply with the provisions of §§ 447. 512(b), 447. 518(a), and 447. 518(d) of the final regulation. • When submitting a SPA, § States must comply with the public notice provisions set forth in § 447. 205. § States must also comply with requirements to solicit advice prior to submission from I/T/U providers pursuant to section 1902(a)(73) of the Act. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 10

Reimbursement Requirements For States • § 447. 518(d) requires that when states propose changes to either the ingredient cost or professional dispensing fee, states must consider both to ensure that total reimbursement to the pharmacy provider is in accordance with requirements of section 1902(a)(30)(A) of the Social Security Act (the Act). This information is only intended to be a general summary and is not a complete or full summary of the final rule. 11

Reimbursement Requirements For States • When proposing reimbursement changes, states are required to submit a state plan amendment (SPA) to CMS for review which includes: – A survey or other reliable data to support any proposed changes to either or both of the components of the reimbursement methodology. – Evidence of having complied with applicable requirements to consult with I/T/U providers pursuant to section 1902(a)(73) of the Act. – Evidence of complying with the public notice provisions set forth in § 447. 205. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 12

Reimbursement for Drugs Purchased Under Other Federal Drug Programs § 447. 518 also requires that the state plan describe the state agency’s payment methodology for prescription drugs, including the agency’s payment methodology for drugs dispensed by all the following: • A covered entity described in section 1927(a)(5)(B) of the Act (340 B covered entity pharmacy). • A contract pharmacy under contract with a 340 B covered entity described in section 1927(a)(5)(B) of the Act. • An Indian Health Service, Tribal and Urban Indian pharmacy (I/T/U). This information is only intended to be a general summary and is not a complete or full summary of the final rule. 13

Reimbursement for Drugs Purchased Under Other Federal Drug Programs • In accordance with the requirements in § 447. 518(a)(2), the state’s payment methodology for drugs dispensed by 340 B covered entities, 340 B contract pharmacies, and I/T/U pharmacies must be in accordance with the definition of AAC in § 447. 502 of the final regulation. § For drugs purchased through the 340 B program, reimbursement should not exceed the 340 B ceiling price. § For drugs purchased outside the 340 B program, the reimbursement should not exceed the provider’s AAC. § For drugs purchased through the FSS, reimbursement should not exceed the FSS price. § States that pay IHS and Tribal providers through encounter rates can continue to pay at that rate since this will satisfy the requirements in § 447. 518(a)(2). This information is only intended to be a general summary and is not a complete or full summary of the final rule. 14

Encounter Rate • The encounter rate is – A uniform amount reimbursed to all I/T/Us by the state for the delivery of any service provided to any patient seen by the facility, irrespective of the nature of the care provided. – Intended to be an all-inclusive average of all provider costs incurred by I/T/Us for the delivery of care for their patients. – Unlike AAC, which is defined in § 447. 502, the encounter rate is more reflective of services provided and is not granular to the extent of identifying the ingredient cost of a drug. – Therefore, if a state pays I/T/Us at the encounter rate, it will satisfy the requirements in § 447. 518(a)(2), which specifies that the state’s payments must be in accordance with the definition of AAC. • Given that encounter rates are designed to address provider costs, we determined that the encounter rate is one model that states may use to reimburse I/T/U pharmacies. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 15

The FUL The Affordable Care Act: • Revised the methodology for calculating a FUL for multiple source drugs as no less than 175% of the weighted average of monthly reported AMPs. • Required that the manufacturers’ total number of units that are used to calculate the monthly AMP be reported – (AMP units). • Modified the confidentiality provisions to allow CMS to disclose the weighted average AMPs on a public website, but not individual AMPs for drug products. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 16

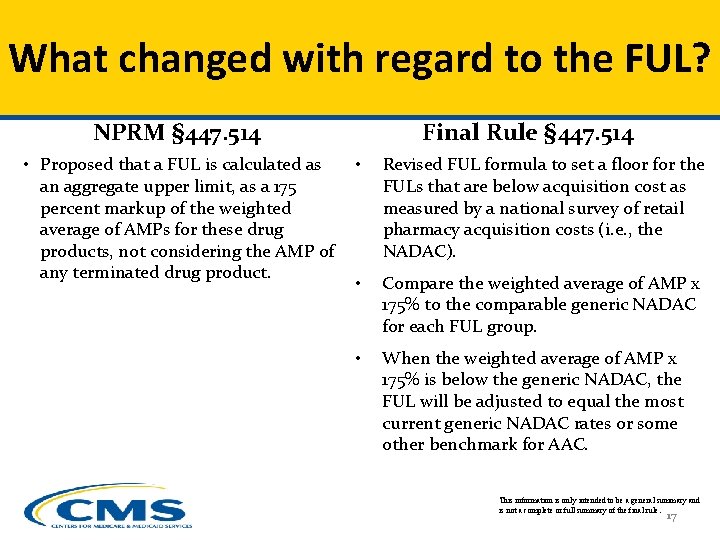
What changed with regard to the FUL? NPRM § 447. 514 • Proposed that a FUL is calculated as an aggregate upper limit, as a 175 percent markup of the weighted average of AMPs for these drug products, not considering the AMP of any terminated drug product. Final Rule § 447. 514 • Revised FUL formula to set a floor for the FULs that are below acquisition cost as measured by a national survey of retail pharmacy acquisition costs (i. e. , the NADAC). • Compare the weighted average of AMP x 175% to the comparable generic NADAC for each FUL group. • When the weighted average of AMP x 175% is below the generic NADAC, the FUL will be adjusted to equal the most current generic NADAC rates or some other benchmark for AAC. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 17

Establishing the FUL • The final rule finalizes the basic criteria for establishing a FUL: § Must be at least three pharmaceutically and therapeutically equivalent (“A rated”), innovator (I) and/or non-innovator (N) multiple source drug products at the NDC-9 level, or product code level, with a monthly AMP and AMP units, greater than zero, reported and certified by the labeler. § Will not include formulations of the drug that are not rated by the Food and Drug Administration (FDA) as pharmaceutically and therapeutically equivalent to the reference listed drug, (A-rated) in the calculation of the weighted average of monthly AMPs, or apply the FUL to those formulations that are not A-rated, e. g. , B-rated drugs. § Will not include the AMP of a terminated drug in the weighted average of monthly AMPs. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 18

Finalization of the FULs • The final Affordable Care Act FULs will be published in late March 2016 and will be effective on April 1, 2016 to coincide with the effective date of the final rule. • States will have up to 30 days from the April 1, 2016 effective date to implement the FULs. • The FULs will be updated on a monthly basis and will be effective the first day of the following month. • Once the final rule is effective, CMS will remove the FULs that are currently in effect, which were last updated on September 25, 2009. • For further information please refer to the ACA FULs Methodology and Data Guide on Medicaid. gov. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 19

Average Manufacturer Price (AMP) and Best Price (BP) In this section we will cover: • Average Manufacture Price (AMP) • Best Price (BP) This information is only intended to be a general summary and is not a complete or full summary of the final rule. 20

AMP Background • AMP is the basis for Medicaid rebates paid by manufacturers and first defined under the Omnibus Budget Reconciliation Act (OBRA) of 1990. • AMP is based, in part, on the price paid by the wholesaler to the manufacturer and certain eligible sales, discounts, rebates, payments, and other financial transactions for drugs sold to community retail pharmacies. • AMP calculation was modified by the Deficit Reduction Act of 2005 (DRA) and the Affordable Care Act of 2010. • DRA mandated that AMP be used as basis for the calculation of the FUL for multiple source drugs. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 21

AMP in the Affordable Care Act The Affordable Care Act: • Included sales to wholesalers and retail community pharmacies and defined them in statute. • Specified transactions for manufacturers to include and exclude in the calculation of AMP. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 22

Excluded* from the Calculation of AMP excludes the following sales, nominal price sales, and associated discounts, rebates, payments, or other financial transactions : (1) Any prices charged under the Federal Supply Schedule (FSS) of the General Services Administration (GSA). (2) Any prices on or after October 1, 1992, to the • Indian Health Service (IHS), • Department of Veterans Affairs (DVA), • Department of Defense (Do. D) • Public Health Service (PHS) • State homes receiving funds under 38 U. S. C. 1741, or • Covered entities described in section 1927(a)(5)(B) of the Act (including inpatient prices charged to hospitals described in section 340 B(a)(4)(L) of the PHSA). * This is not an exhaustive list. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 23

Best Price (BP) Background Best price for a drug of a manufacturer means the lowest price available from the manufacturer during the rebate period to any wholesaler, retailer, provider, health maintenance organization, nonprofit entity, or governmental entity in the United States in any pricing structure (including capitated payments), in the same quarter for which the AMP is computed. This information is only intended to be a general summary and is not a complete or full summary of the final rule. 24

Prices Excluded* from BP Any prices charged: • To the IHS, the DVA, a State home receiving funds under 38 U. S. C. 1741, the Do. D, or the PHS on or after October 1, 1992, charged to • To a covered entity described in section 1927(a)(5)(B) of the Act (including inpatient prices charged to hospitals described in section 340 B(a)(4)(L) of the PHSA) • Under the FSS. *This is not an exhaustive list This information is only intended to be a general summary and is not a complete or full summary of the final rule. 25

Questions? Questions about the final rule with comment should be sent to rxdrugpolicy@cms. hhs. gov with the question’s topic in the Subject line (e. g. Final Rule with comment - FULs). This information is only intended to be a general summary and 26 is not a complete or full summary of the final rule.