Mechanisms of Toxicity of Oil Sands Process Affected





- Slides: 19

Mechanism(s) of Toxicity of Oil Sands Process Affected Water Steve Wiseman Toxicology Centre University of Saskatchewan

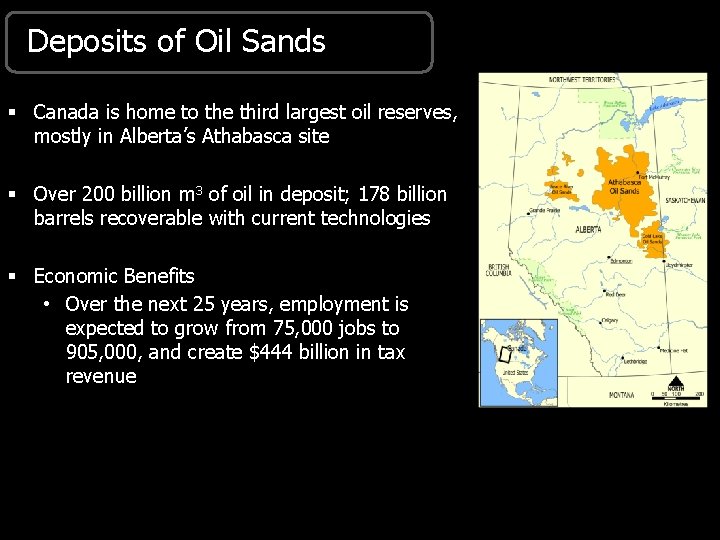
Deposits of Oil Sands § Canada is home to the third largest oil reserves, mostly in Alberta’s Athabasca site § Over 200 billion m 3 of oil in deposit; 178 billion barrels recoverable with current technologies § Economic Benefits • Over the next 25 years, employment is expected to grow from 75, 000 jobs to 905, 000, and create $444 billion in tax revenue

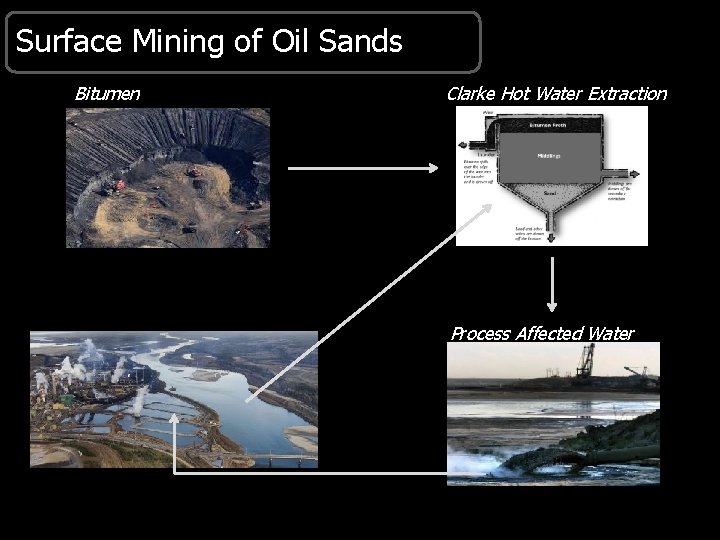
Surface Mining of Oil Sands Bitumen Clarke Hot Water Extraction Process Affected Water

Oil Sand Process-Affected Water (OSPW) § Oil Sands Process-affected Water (OSPW) • Sands, clay, metals, unrecoverable bitumen • Polycyclic aromatic hydrocarbons (PAHs; particle bound) • Dissolved organic fraction containing >250, 000 chemicals, including naphthenic acids (NAs) • Held in on-site tailings ponds under a policy of no release

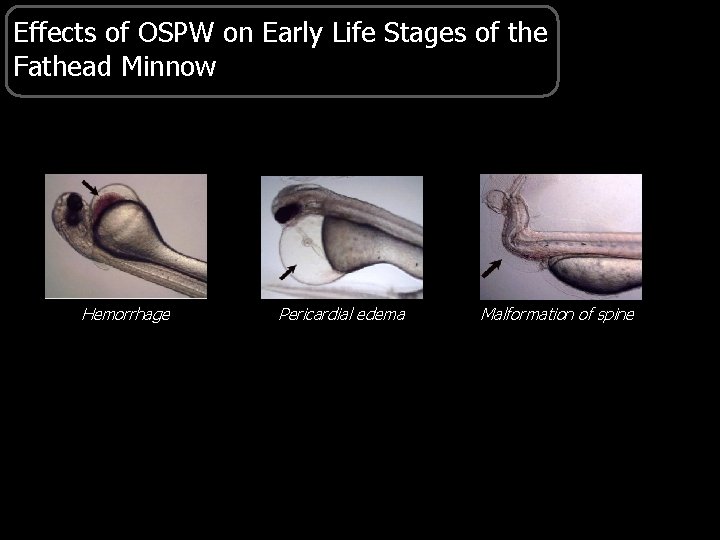
Effects of OSPW on Aquatic Organisms § Endocrine disruption • Changes in concentrations of T and E 2 • Impaired reproduction of fathead minnows exposed to OSPW § Embryotoxicity • Reduced growth • Greater mortality, hemorrhages, malformations • Greater EROD activity (sediment/tailings) § Immunotoxicity • Greater incidences of fin erosion and viral lesions • Decreases leukocytes, thrombocytes, and granulocytes

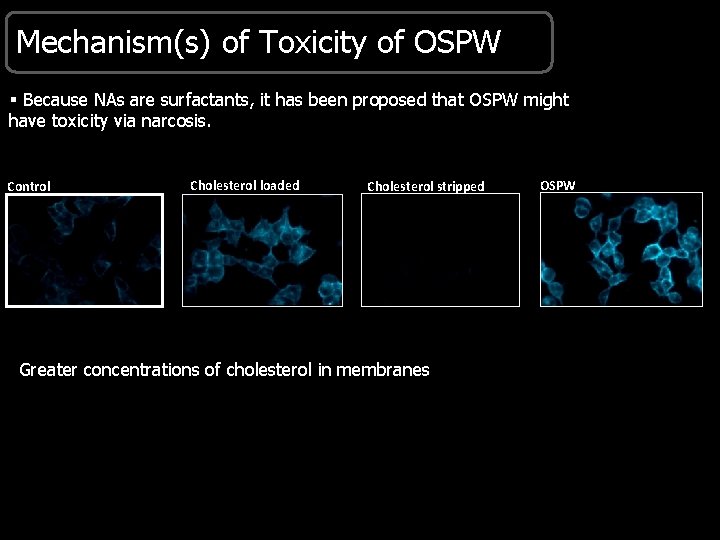
Mechanism(s) of Toxicity of OSPW § Because NAs are surfactants, it has been proposed that OSPW might have toxicity via narcosis. Control Cholesterol loaded Cholesterol stripped Greater concentrations of cholesterol in membranes OSPW

Transcriptomics Given the complexity of OSPW it is likely that there are multiple mechanisms of toxicity. Goal: Quantify abundances of transcripts in livers of male fathead minnows exposed to OSPW to gain insight into potential mechanisms of toxicity.

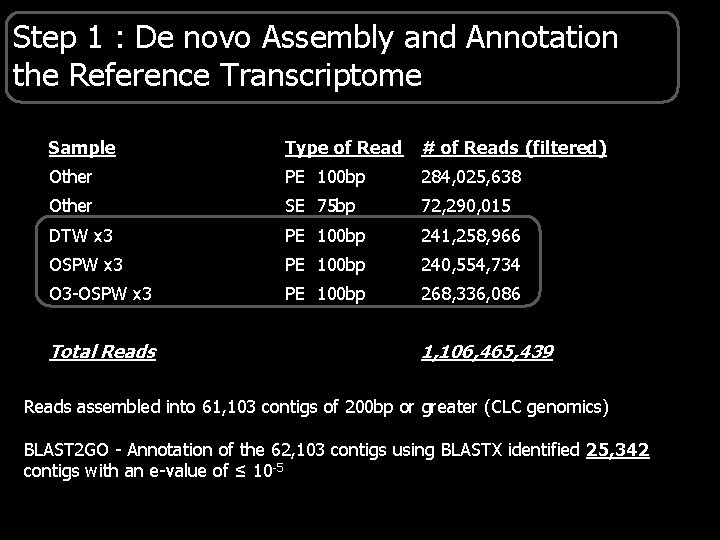
Step 1 : De novo Assembly and Annotation the Reference Transcriptome Sample Type of Read # of Reads (filtered) Other PE 100 bp 284, 025, 638 Other SE 75 bp 72, 290, 015 DTW x 3 PE 100 bp 241, 258, 966 OSPW x 3 PE 100 bp 240, 554, 734 O 3 -OSPW x 3 PE 100 bp 268, 336, 086 Total Reads 1, 106, 465, 439 Reads assembled into 61, 103 contigs of 200 bp or greater (CLC genomics) BLAST 2 GO - Annotation of the 62, 103 contigs using BLASTX identified 25, 342 contigs with an e-value of ≤ 10 -5

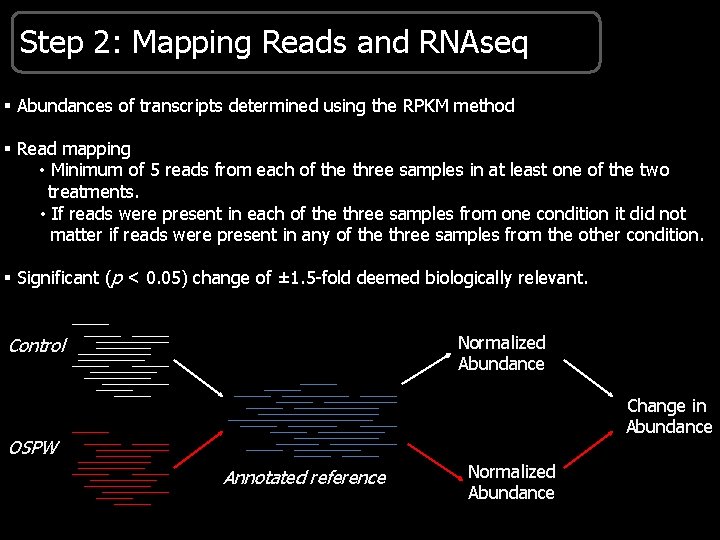
Step 2: Mapping Reads and RNAseq § Abundances of transcripts determined using the RPKM method § Read mapping • Minimum of 5 reads from each of the three samples in at least one of the two treatments. • If reads were present in each of the three samples from one condition it did not matter if reads were present in any of the three samples from the other condition. § Significant (p < 0. 05) change of ± 1. 5 -fold deemed biologically relevant. Normalized Abundance Control Change in Abundance OSPW Annotated reference Normalized Abundance

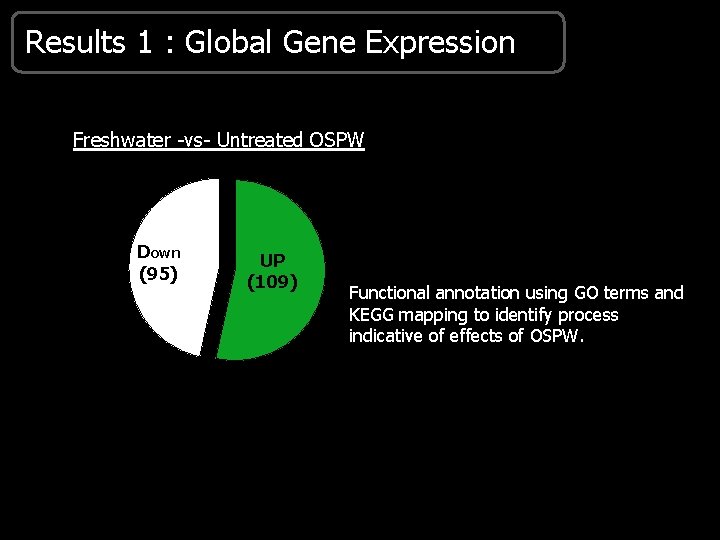
Results 1 : Global Gene Expression Freshwater -vs- Untreated OSPW Down (95) UP (109) Functional annotation using GO terms and KEGG mapping to identify process indicative of effects of OSPW.

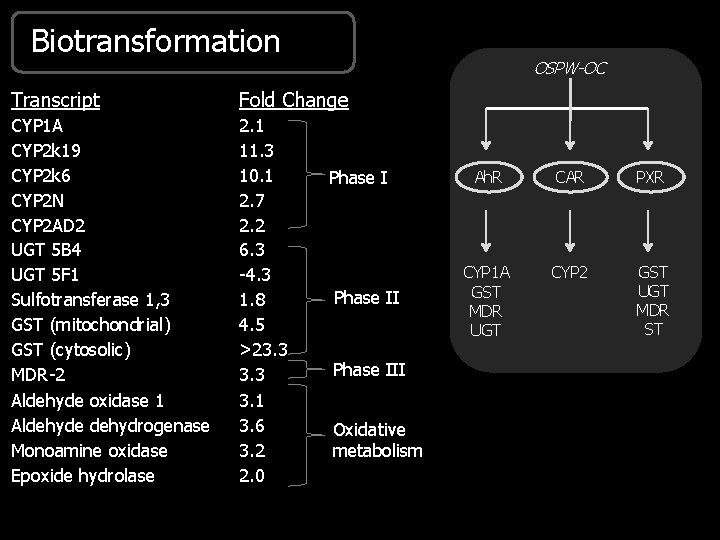
Biotransformation OSPW-OC Transcript Fold Change CYP 1 A CYP 2 k 19 CYP 2 k 6 CYP 2 N CYP 2 AD 2 UGT 5 B 4 UGT 5 F 1 Sulfotransferase 1, 3 GST (mitochondrial) GST (cytosolic) MDR-2 Aldehyde oxidase 1 Aldehyde dehydrogenase Monoamine oxidase Epoxide hydrolase 2. 1 11. 3 10. 1 2. 7 2. 2 6. 3 -4. 3 1. 8 4. 5 >23. 3 3. 1 3. 6 3. 2 2. 0 Phase III Oxidative metabolism Ah. R CAR PXR CYP 1 A GST MDR UGT CYP 2 GST UGT MDR ST

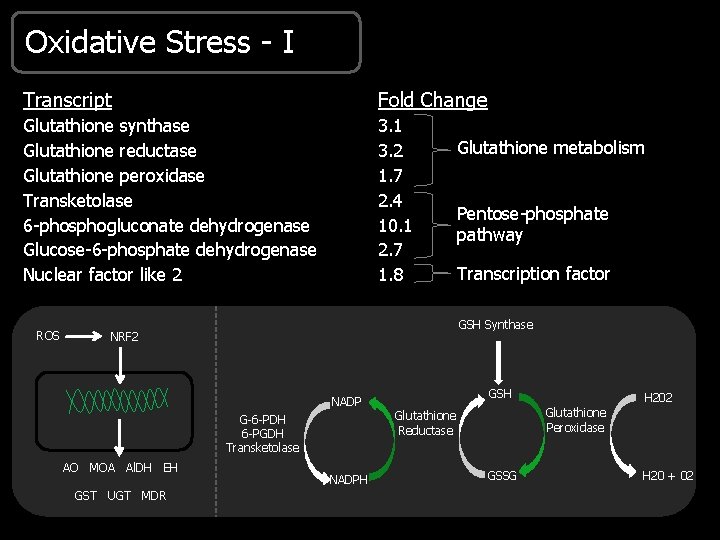
Oxidative Stress - I Transcript Fold Change Glutathione synthase Glutathione reductase Glutathione peroxidase Transketolase 6 -phosphogluconate dehydrogenase Glucose-6 -phosphate dehydrogenase Nuclear factor like 2 3. 1 3. 2 1. 7 2. 4 10. 1 2. 7 1. 8 ROS Transcription factor GSH NADPH H 202 Glutathione Peroxidase Glutathione Reductase G-6 -PDH 6 -PGDH Transketolase GST UGT MDR Pentose-phosphate pathway GSH Synthase NRF 2 AO MOA Al. DH EH Glutathione metabolism GSSG H 20 + 02

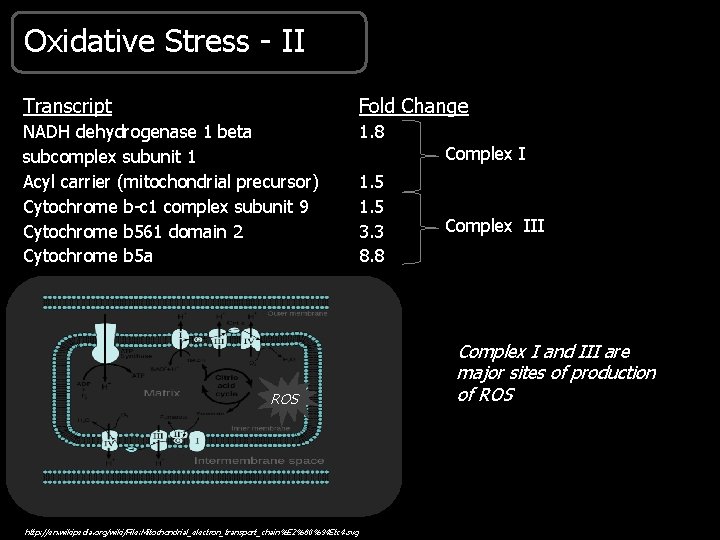
Oxidative Stress - II Transcript Fold Change NADH dehydrogenase 1 beta subcomplex subunit 1 Acyl carrier (mitochondrial precursor) Cytochrome b-c 1 complex subunit 9 Cytochrome b 561 domain 2 Cytochrome b 5 a 1. 8 ROS http: //en. wikipedia. org/wiki/File: Mitochondrial_electron_transport_chain%E 2%80%94 Etc 4. svg Complex I 1. 5 3. 3 8. 8 Complex III Complex I and III are major sites of production of ROS

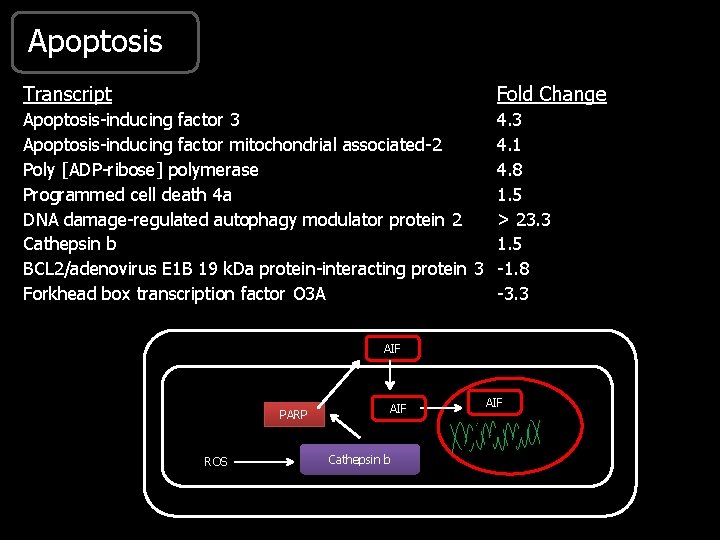
Apoptosis Transcript Fold Change Apoptosis-inducing factor 3 4. 3 Apoptosis-inducing factor mitochondrial associated-2 4. 1 Poly [ADP-ribose] polymerase 4. 8 Programmed cell death 4 a 1. 5 DNA damage-regulated autophagy modulator protein 2 > 23. 3 Cathepsin b 1. 5 BCL 2/adenovirus E 1 B 19 k. Da protein-interacting protein 3 -1. 8 Forkhead box transcription factor O 3 A -3. 3 AIF PARP ROS AIF Cathepsin b AIF

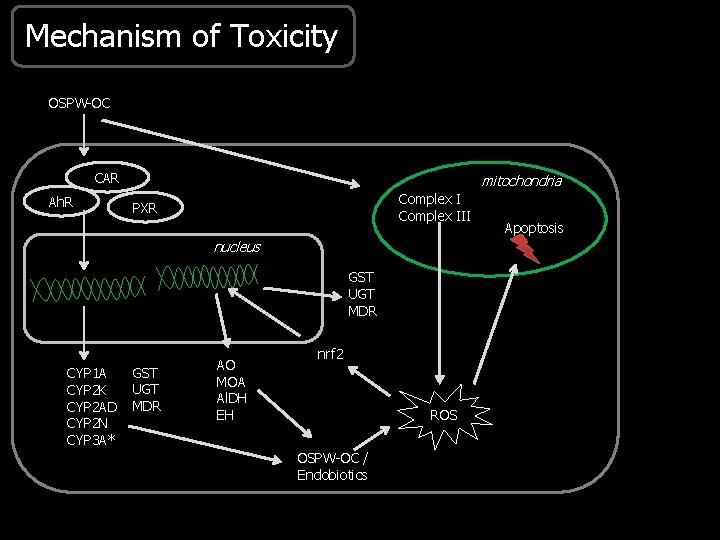
Mechanism of Toxicity OSPW-OC CAR Ah. R mitochondria Complex III PXR nucleus GST UGT MDR CYP 1 A CYP 2 K CYP 2 AD CYP 2 N CYP 3 A* GST UGT MDR AO MOA Al. DH EH nrf 2 ROS OSPW-OC / Endobiotics Apoptosis

Effects of OSPW on Early Life Stages of the Fathead Minnow Hemorrhage Pericardial edema Malformation of spine

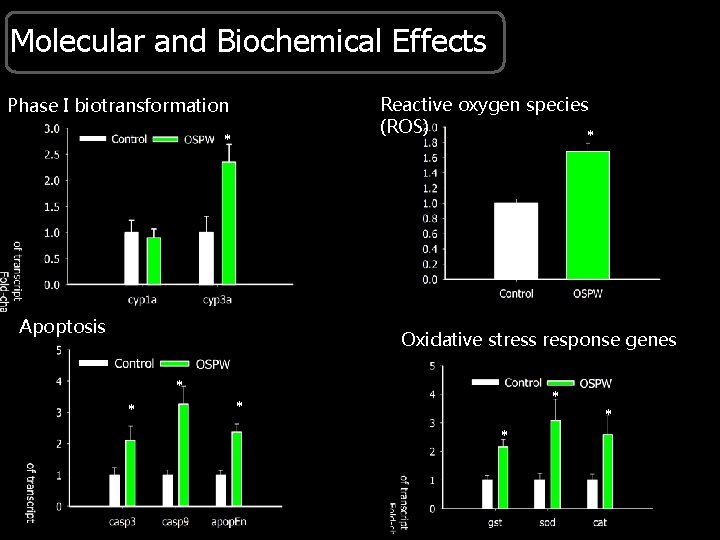
Molecular and Biochemical Effects Reactive oxygen species (ROS) * Phase I biotransformation * Apoptosis Oxidative stress response genes * * *

Conclusions § RNAseq - apoptosis induced by ROS that result from metabolism of organic compounds in OSPW and from changes in mitochondrial respiration might cause toxicity of OSPW. § Results of the RNAseq are supported by results from embryotoxicity of OSPW. § Abundances determined by RNAseq match changes determined by q. PCR. Where next ? § What are the chemicals in OSPW that are causing these effects? §Targeted studies to further establish this mechanism of toxicity. §Development of a PCR array.

John Giesy Yuhe He Rishi Mandinky Markus Hecker Paul Jones Sarah Peterson Warren Zubot Jon Martin Mohamed Gamal El-Din