Mechanisms of cell injury Hussam Telfah MBBS FRCPath

Mechanisms of cell injury Hussam Telfah, MBBS, FRCPath
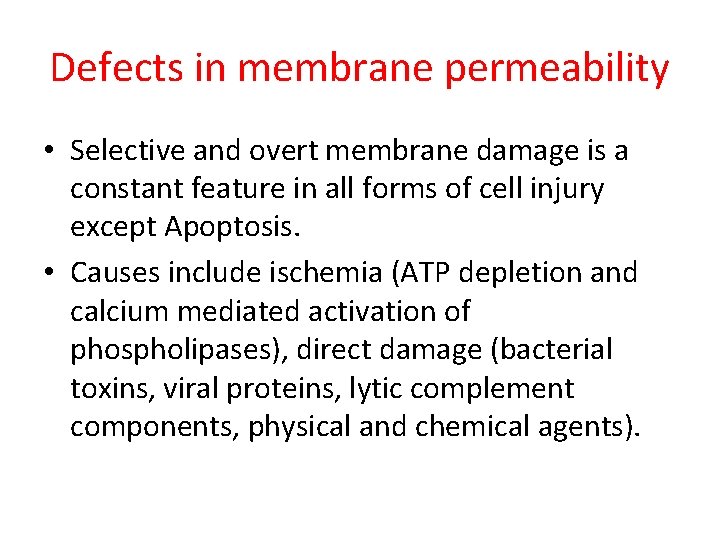
Defects in membrane permeability • Selective and overt membrane damage is a constant feature in all forms of cell injury except Apoptosis. • Causes include ischemia (ATP depletion and calcium mediated activation of phospholipases), direct damage (bacterial toxins, viral proteins, lytic complement components, physical and chemical agents).
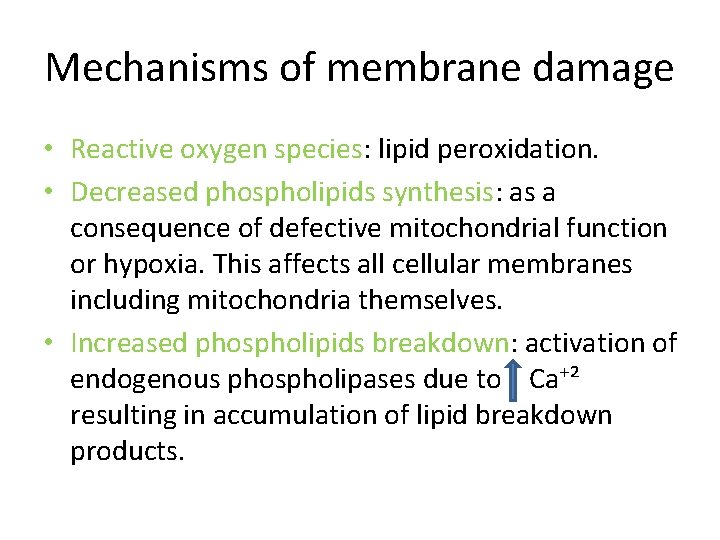
Mechanisms of membrane damage • Reactive oxygen species: lipid peroxidation. • Decreased phospholipids synthesis: as a consequence of defective mitochondrial function or hypoxia. This affects all cellular membranes including mitochondria themselves. • Increased phospholipids breakdown: activation of endogenous phospholipases due to Ca⁺² resulting in accumulation of lipid breakdown products.
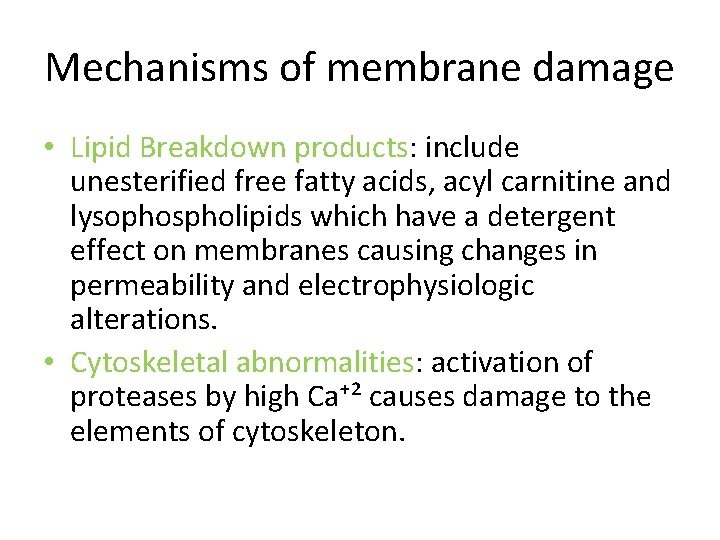
Mechanisms of membrane damage • Lipid Breakdown products: include unesterified free fatty acids, acyl carnitine and lysophospholipids which have a detergent effect on membranes causing changes in permeability and electrophysiologic alterations. • Cytoskeletal abnormalities: activation of proteases by high Ca⁺² causes damage to the elements of cytoskeleton.

Consequences of membrane damage • Most important sites of membrane damage: mitochondrial, plasma membrane and lysosomal. • Lysosomes contain many degrading enzymes like RNases, DNases, proteases. . .
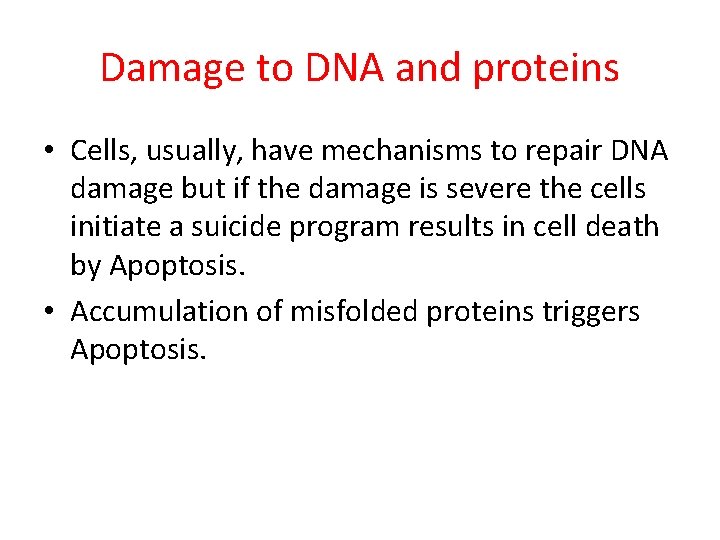
Damage to DNA and proteins • Cells, usually, have mechanisms to repair DNA damage but if the damage is severe the cells initiate a suicide program results in cell death by Apoptosis. • Accumulation of misfolded proteins triggers Apoptosis.
Concluding points • The identification of factors that determine when reversible injury becomes irreversible and progresses to cell death would be very useful so we may be able to identify strategies to prevent permanent consequences of cell injury. • Leakage of intracellular proteins into blood through damaged membranes provides a means of detecting tissue damage. CK & troponin in MI and ALT, AST &ALK in liver.

Clinico. Pathologic Correlation

Ischemic and hypoxic injury • Most common type of injury in clinical medicine. • Hypoxia: anaerobic glycolysis • Ischemia: delivery of substrates is also compromise. • Ischemia is more rapidly damaging than hypoxia in the absence of ischemia.
Mechanisms of ischemic injury • Low O 2 leads to loss of oxidative phosphorylation and decreased generation of ATP. • Na/K and Ca⁺² pumps failure. • Progressive loss of glycogen and decreased protein synthesis. • Loss of function though the cell is not yet dead.
Mechanisms of ischemic injury • Cytoskeleton abnormalities; blebs and loss of villi. • Formation of myelin figures and swollen organelles. • To this point changes are reversible. • After that, severe swelling to the mitochondria, extensive damage to the plasma membranes, myelin figures formation and swelling of lysosomes.
Mechanisms of ischemic injury • Large densities develop in the mitochondria. • Massive influx of Ca⁺² happens especially if the ischemic area is reperfused. • Death is mainly by necrosis but apoptosis also takes place. • Dead cells may become replaced by large masses of myelin figures which are either phagocytosed or degraded more into fatty acids and may become calcified.
Mechanisms of ischemic injury • Protective responses: Hypoxia-inducible factor -1; promotes new blood vessel formation, stimulates cell survival pathways and enhances anaerobic glycolysis. • Still no reliable therapeutic measure to reduce consequences of ischemia clinically. • Induction of hypothermia (33. 4⁰) in ischemic brain and spinal injuries may help in reducing the effects of injury.
Ischemia-reperfusion injury • Restoration of blood flow to ischemic tissues can promote recovery if they are reversibly injured. • In certain situations, reperfusion paradoxically exacerbates injury (more dead cells in addition to the already irreversibly injured cells). • Mechanisms:
Ischemia- reperfusion injury • Reoxygenation: increased regeneration of reactive oxygen and nitrogen species from parenchymal and endothelial cells and leukocytes. Ca⁺² influx. • Inflammation response mediated by cytokines which recruits more leukocytes and more injury. Applying of Anti-cytokines might aid in decreasing the unwanted effects of inflammation.

Ischemia- reperfusion injury • Activation of the complement system: Some Ig. M antibodies are deposited in ischemic tissues for unknown reasons and once the blood is restored complement proteins bind to those antibodies and lead to their activation and so more injury.
Chemical injury • Major problem. Drugs. • Liver as a major site of drug metabolism is a target for drug toxicity. • Mechanisms: • Directly by combining with critical molecular component. Example mercuric chloride poisoning bind to the sulfhydryl groups of cell membrane proteins causing increased permeability. More in GIT and kidney.
Chemical injury Cyanide poisons mitochondrial cytochrome oxidase and inhibits oxidative phosphorylation • Most chemicals are not biologically active and need to be converted into active forms (toxic metabolites) which usually takes place in liver ( cytochrome P-450 mixed-function oxidases). Free radical formation and lipid peroxidation. CCl 4 is converted to CCl 3 • which causes lipid peroxidation and decrease export of lipids (Fatty change).

Chemical injury Acetaminophen (paracetamol) converted to to toxic products in liver leading to injury.

Apoptosis • Pathway of cell death induced by a suicide program in which activation of degrading enzymes takes place. • Apoptotic cells break up into fragments called apoptotic bodies which contain portions of the cytoplasm and nucleus. Become targets for phagocytosis before their contents leak out and so there would be no inflammatory reaction.
Apoptosis • Occurs normally during development and adulthood and in pathologic conditions. • Physiologic situations: Embryogenesis, involution of hormonedependent tissues upon hormone withdrawal, cell loss in proliferating cell populations and death of host cells after serving their useful function.
Apoptosis • Pathologic situations: (no host reaction) DNA damage, accumulation of misfolded proteins (Excessive accumulation of these proteins in the ER called ER stress), certain infections (viral ones), pathologic atrophy in parenchymal organs after duct obstruction (pancreas, parotid and kidney)
- Slides: 22