Mechanism of hormone action Hormones Three types Proteins








- Slides: 29

Mechanism of hormone action

Hormones • Three types – Proteins • Glycoproteins • Small pepstides • Large proteins – Lipids • Cholesterol derivatives • Eicosanoids – Amino acid derivatives

• Hormones – Innate by themselves – Require mediation • Receptors – Binding sites for a hormone • Very specific

Hormone receptors • Two types – Transmembrane – Intracellular/nuclear – Proteins regardless of the type • Interaction between a hormone and a receptor – Initial step of hormone action

Transmembrane receptors • Protein hormones – Unable to pass through the plasma membrane • Size • Charges – Receptors must be located on the plasma membrane • Extracellular domain for interaction with hormone • Intracellular signaling system

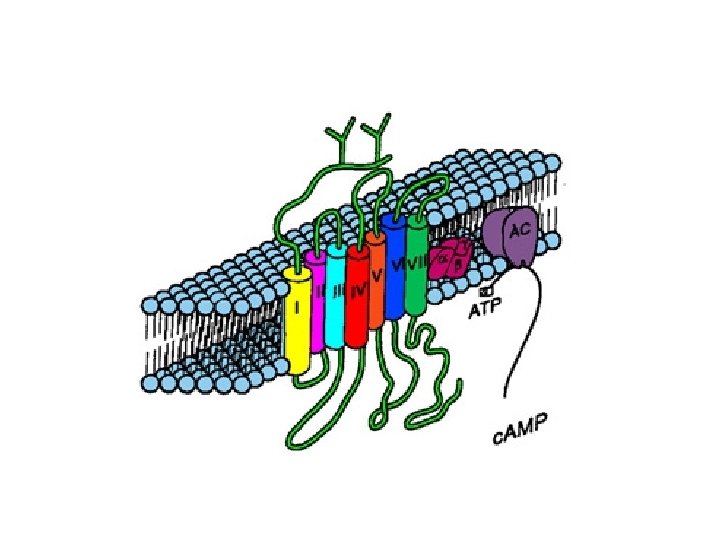
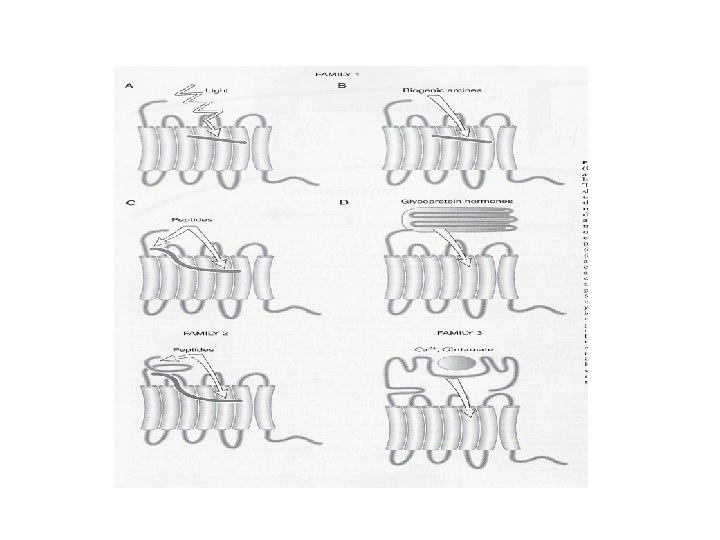
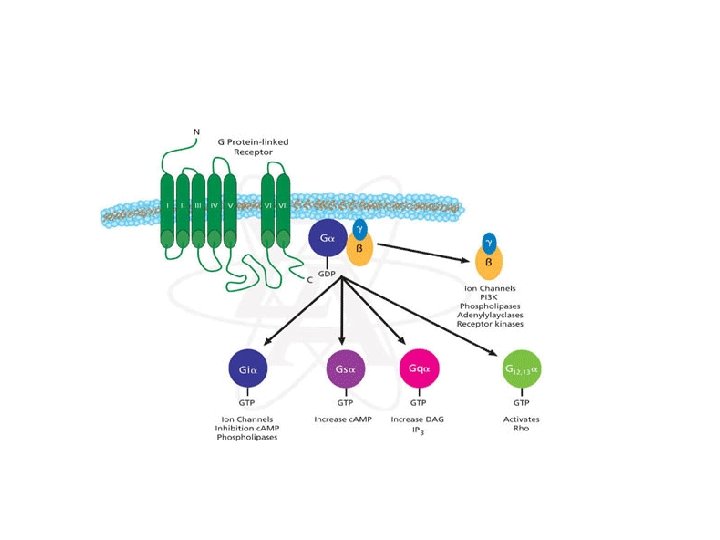
Types of transmembrane receptors • Receptors with multiple transmembrane domains – Seven trans-membrane domain receptor – No intrinsic enzymatic activity (C-terminus) • Associated with intracellular proteins involved in signaling – G-proteins – Modification of extracellular domain (hormone binding site, N-terminus) • Glycosylation – Crucial for hormone binding


• Trans-membrane domains (7) – Alpha-helix • Hydrophobic amino acids • Loops – Connect alpha helices • May be linked by disulfide bridges (extracellular loop 1 and 2)

• Intracellular/cytoplasmic domain – Palmitoylation of some cysteine residues • Attachment of fatty acids • Fourth loop – Site for phosphorylation

• General structure of seven transmembrane receptor – Variations • Amino acid sequences – Variable length of N-terminus – Affects binding of ligand/hormone


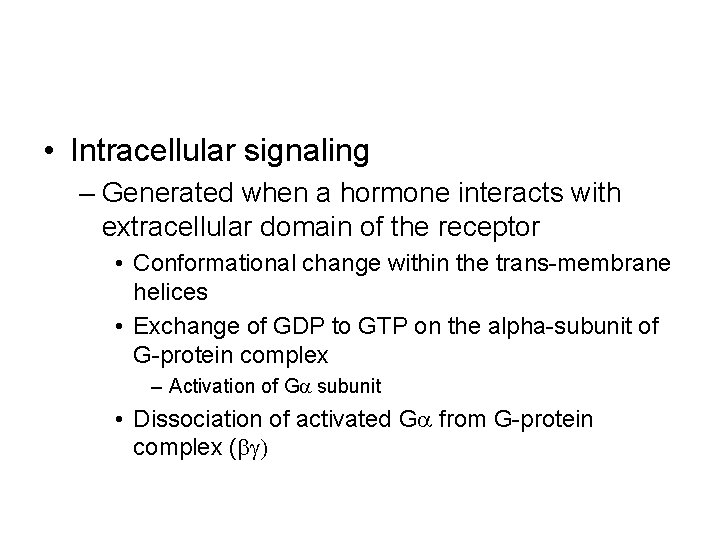
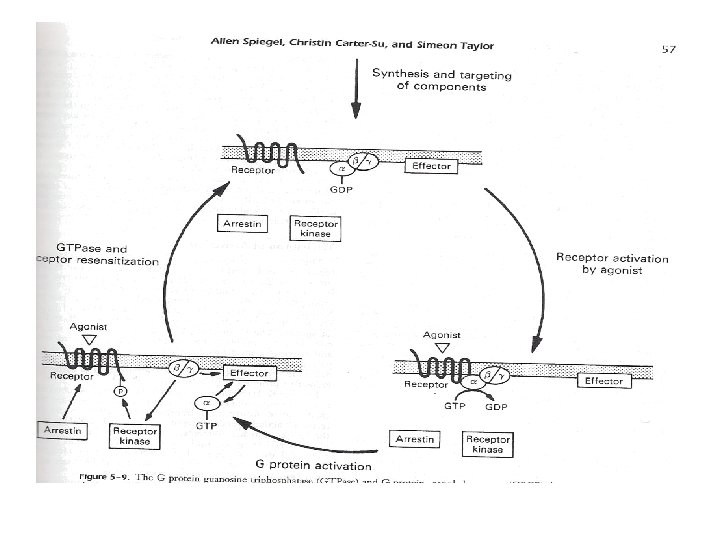
• Intracellular signaling – Generated when a hormone interacts with extracellular domain of the receptor • Conformational change within the trans-membrane helices • Exchange of GDP to GTP on the alpha-subunit of G-protein complex – Activation of Ga subunit • Dissociation of activated Ga from G-protein complex (bg)


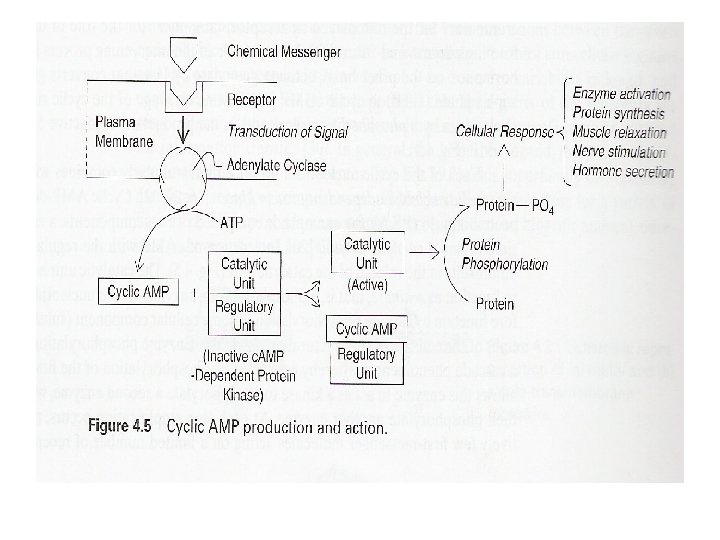
• Second messengers – Cyclic nucleotides (c. AMP and c. GMP) • c. AMP – Widely used secondary messenger – Generated by adenyl cyclase » Activated by activated Ga subunit of G-protein complex • Activation of cyclic nucleotide-dependent protein kinases – Protein kinase A (c. AMP)

• Secondary messengers – Amplification of hormonal signals • Binding of hormone to the receptor • Activation of adenyl cyclase by activated Ga • Activation of protein kinase A by c. AMP – Rapid clearance and inactivation • Phosphodiesterases – Inhibited by methylxanthines (caffeine, theophylline, and theobromine) • Phosphoprotein phosphatases


• How do we know that c. AMP is a secondary messenger? – Changes in production of c. AMP after hormonal treatment – Correlation between amount of c. AMP being produced and cellular response to the hormone – Inhibition of phosphodiesterase activity • Presence of ligand but no effects – Treatment with c. AMP analogues/agonists • Similar response to that of hormone

• Types of G-protein complex – Ga subunit (20 different types) • • Gs (stimulatory Ga) Gi (inhibitory Ga) Go (associated with orphan receptors in neurons) Gt (transducin found in retina, activates c. GMPspecific phosphodiesterases) – bg complex • 4 or more


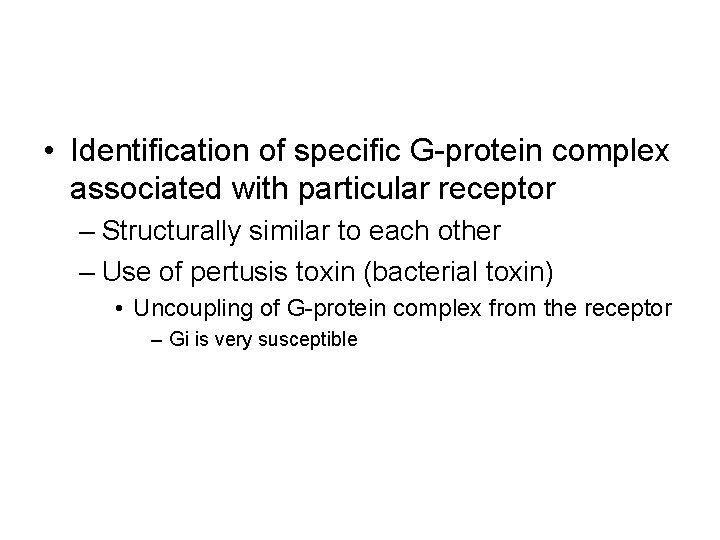
• Identification of specific G-protein complex associated with particular receptor – Structurally similar to each other – Use of pertusis toxin (bacterial toxin) • Uncoupling of G-protein complex from the receptor – Gi is very susceptible

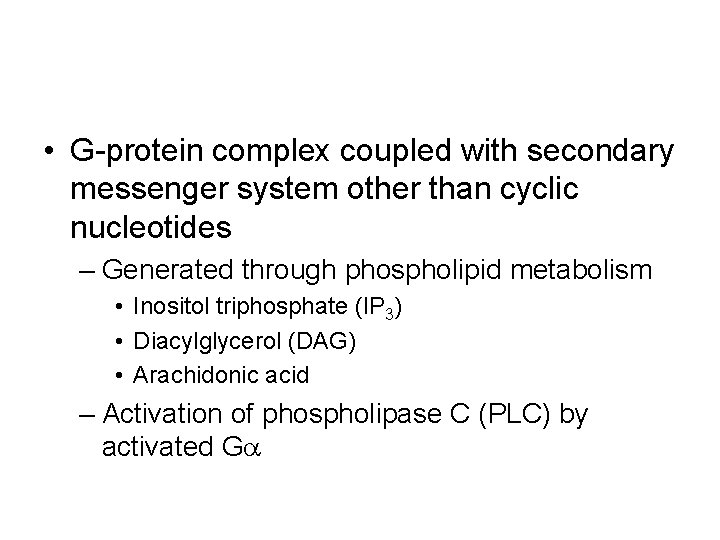
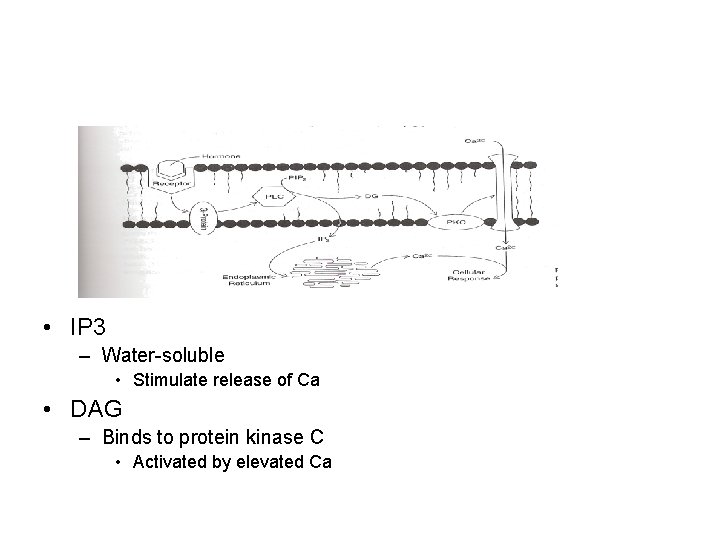
• G-protein complex coupled with secondary messenger system other than cyclic nucleotides – Generated through phospholipid metabolism • Inositol triphosphate (IP 3) • Diacylglycerol (DAG) • Arachidonic acid – Activation of phospholipase C (PLC) by activated Ga

• IP 3 – Water-soluble • Stimulate release of Ca • DAG – Binds to protein kinase C • Activated by elevated Ca

• Medical importance – 65 % of prescription drugs target G-protein coupled receptors • Variety of ligands

Other protein hormone receptors • Transmembrane receptors with intrinsic tyrosine kinase activity – Receptor tyrosine kinase • Receptors for insulin and many growth factors • Transmembrane receptors with associated tyrosine kinases – Cytokine receptors • Receptors for growth hormone and prolactin • No intrinsic kinase activity • Interaction between receptor and hormone causes recruitment and activation of tyrosine kinases associated with receptor

Receptor tyrosine kinase • Approximately 100 receptor tyrosine kinases in human – Highly conserved • Domains – Extracellular • Hormone binding site – Transmembrane – Intracellular/cytoplasmic • Tyrosine kinase activity

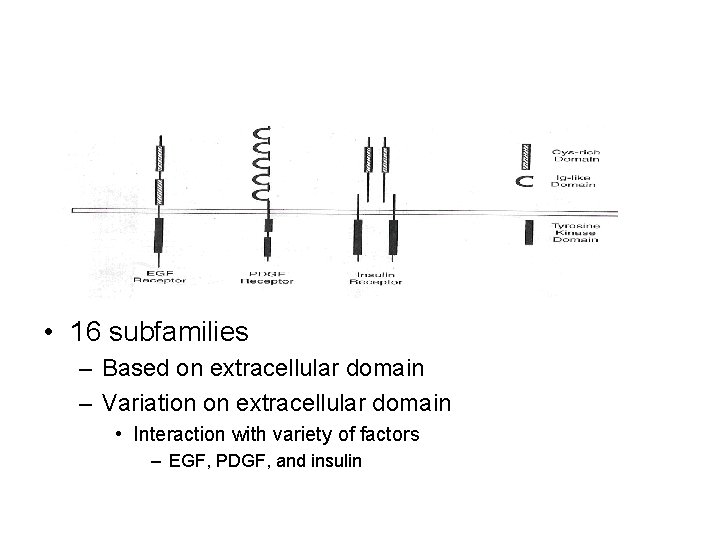
• 16 subfamilies – Based on extracellular domain – Variation on extracellular domain • Interaction with variety of factors – EGF, PDGF, and insulin

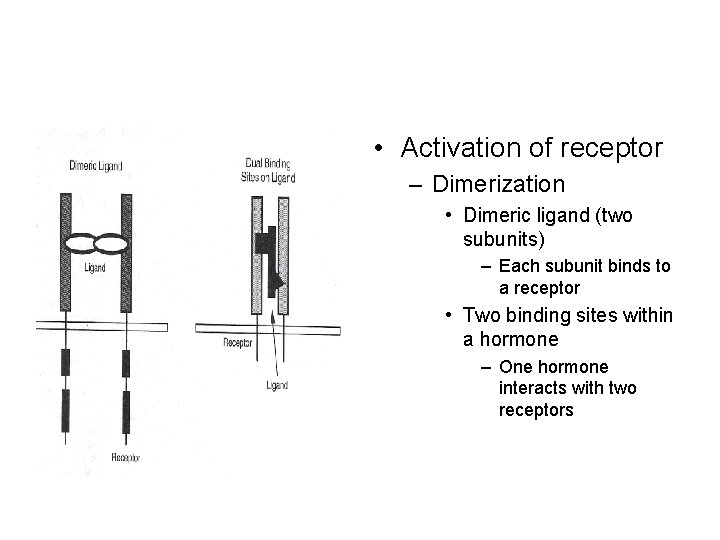
• Activation of receptor – Dimerization • Dimeric ligand (two subunits) – Each subunit binds to a receptor • Two binding sites within a hormone – One hormone interacts with two receptors

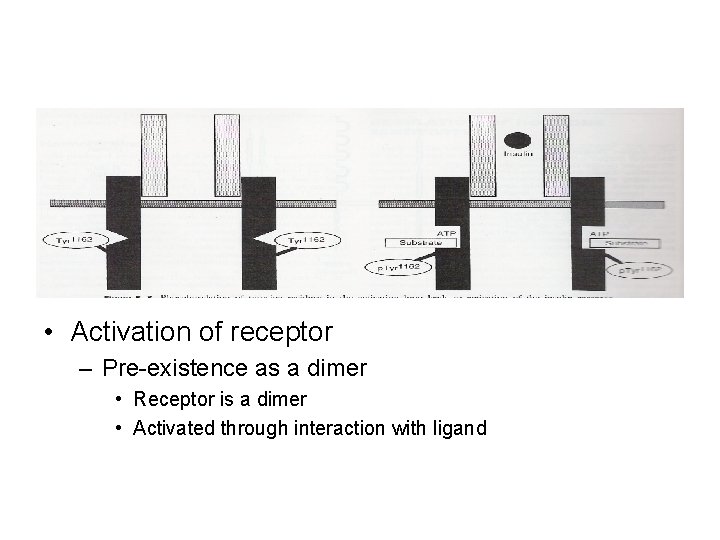
• Activation of receptor – Pre-existence as a dimer • Receptor is a dimer • Activated through interaction with ligand

• Activation of receptor – Conformational changes in the kinase domain • Accessible to the substrate – Autophosphorylation of tyrosine residues (3 in insulin receptor) • Activation loop • Triggers conformational changes – ATP binding – Interaction with intracellular proteins – Phosphorylation of other proteins