Mechanics of respiration Surface Tension and its Biomedical
Mechanics of respiration Surface Tension and its Biomedical Imprtance 4. 12
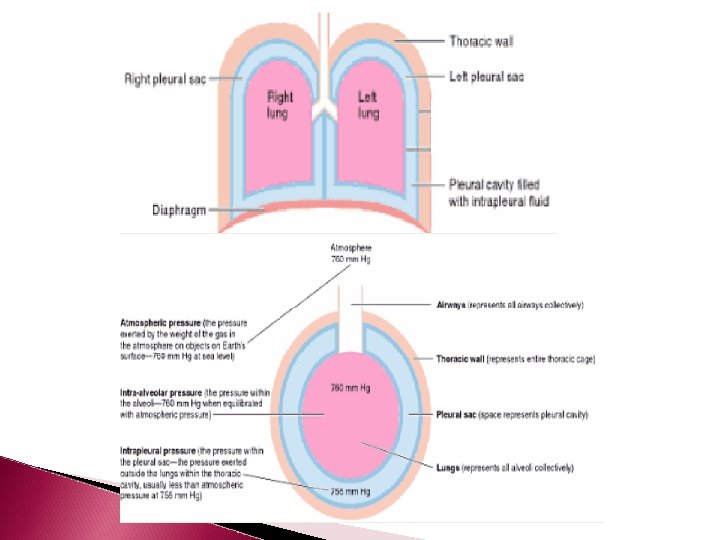

Importance of intrapleural Fluid Cohesiveness When thorax expands during inhalation due to muscular activity in diaphragm, so do the lungs and when thorax contracts so do the lungs during exhalation This is because of the intrapleural fluid cohesiveness and transmural pressure gradient The water molecules in the intrapleural fluid resist being pulled apart because they are polar. This tends to bind the pleural surfaces together
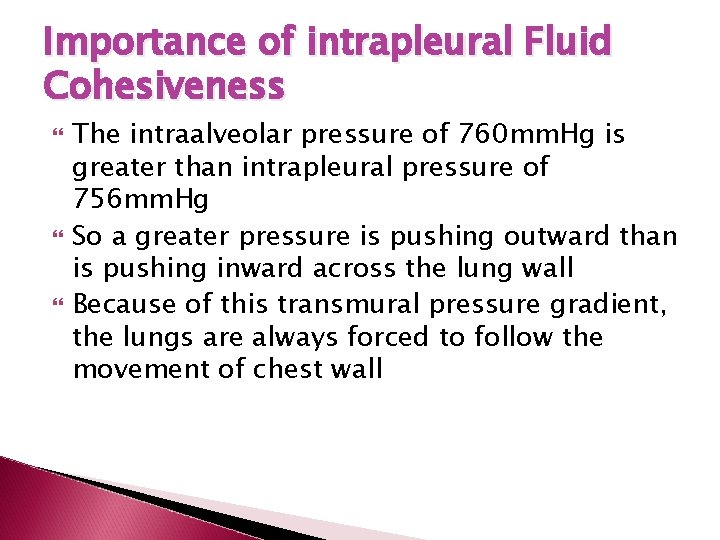
Importance of intrapleural Fluid Cohesiveness The intraalveolar pressure of 760 mm. Hg is greater than intrapleural pressure of 756 mm. Hg So a greater pressure is pushing outward than is pushing inward across the lung wall Because of this transmural pressure gradient, the lungs are always forced to follow the movement of chest wall
Pressure gradient responsible for air flow into or out of alveoli Flow of air into or out of the lungs occurs because of cyclic changes in intra alveolar pressure For air to flow in, intraalveolar pressure must be less than atmospheric pressure during inhalation and vice versa for exhalation
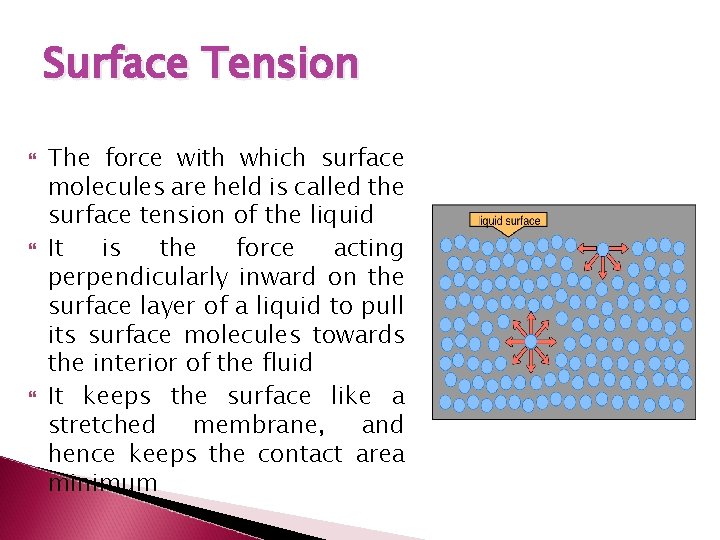
Surface Tension The force with which surface molecules are held is called the surface tension of the liquid It is the force acting perpendicularly inward on the surface layer of a liquid to pull its surface molecules towards the interior of the fluid It keeps the surface like a stretched membrane, and hence keeps the contact area minimum

Surface Tension

Factors affecting surface tension Temperature Solutes can have different effects on surface tension depending on their structure: Little or no effect, for example sugar Increase surface tension, inorganic salts Decrease surface tension progressively, alcohols Decrease surface tension and, once a minimum is reached, no more effect: surfactants

Gibbs-Thomsan Principle According to this principle substances which lower the surface tension becomes concentrated in the surface layer whereas substances which increase surface tension are distributed in the interior of the liquid Soaps and bile salts reduce the surface tension of water while sodium chloride and most inorganic salts increase the surface tension

Biomedical Importance of surface tension Surface tension of plasma is slightly less than water Emulsifying action of Bile salts are surface active. . Bile salts lowers surface tension of fat droplets breaking them into smaller droplets, thereby increasing surface area. This facilitates action of pancreatic lipase Hay’s test This test is based on the surface tension and is employed for detecting the presence of bile salts in urine (an indication of jaundice). If urine contains bile salts, the fine sulfur powder sprinkled on its surface settles down due to lowering of surface tension. Fine sulfur continues to float on the surface if urine does not contain bile salts

Role of surface tension and surfactant in respiration
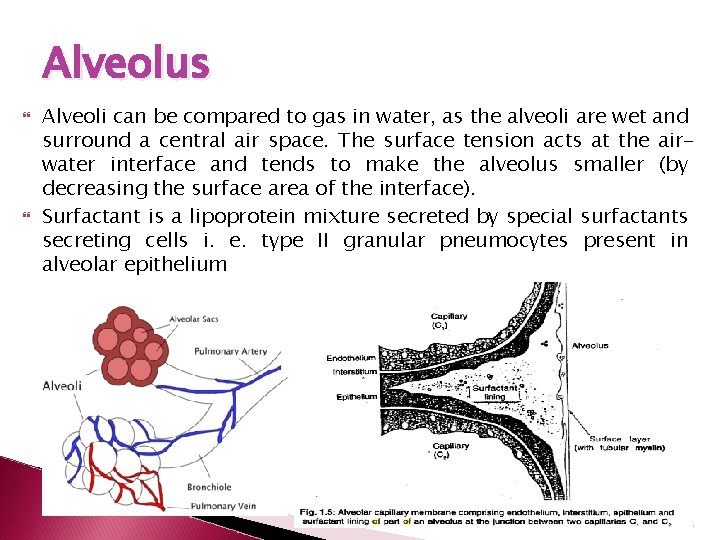
Alveolus Alveoli can be compared to gas in water, as the alveoli are wet and surround a central air space. The surface tension acts at the airwater interface and tends to make the alveolus smaller (by decreasing the surface area of the interface). Surfactant is a lipoprotein mixture secreted by special surfactants secreting cells i. e. type II granular pneumocytes present in alveolar epithelium
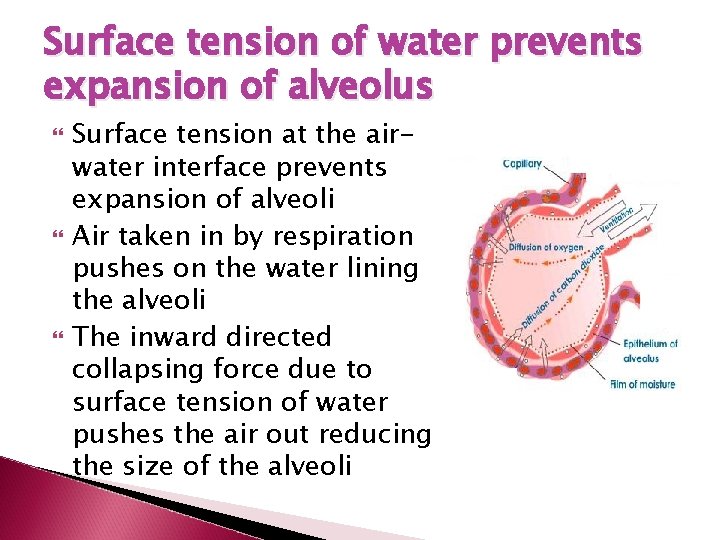
Surface tension of water prevents expansion of alveolus Surface tension at the airwater interface prevents expansion of alveoli Air taken in by respiration pushes on the water lining the alveoli The inward directed collapsing force due to surface tension of water pushes the air out reducing the size of the alveoli

Laplace law For an alveolus, Laplace law can be stated as “The magnitude of inward directed collapsing pressure inside a bubble is inversely proportional to the radius of the bubble and directly proportional to the surface tension at the airwater interface”
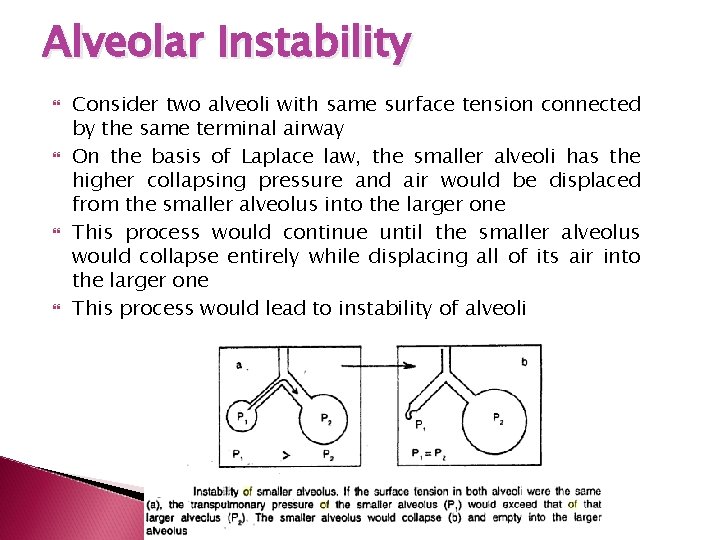
Alveolar Instability Consider two alveoli with same surface tension connected by the same terminal airway On the basis of Laplace law, the smaller alveoli has the higher collapsing pressure and air would be displaced from the smaller alveolus into the larger one This process would continue until the smaller alveolus would collapse entirely while displacing all of its air into the larger one This process would lead to instability of alveoli
- Slides: 15