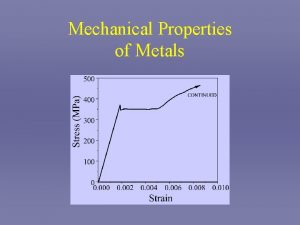
Mechanical properties of DNA under stretching Why important







- Slides: 31

Mechanical properties of DNA under stretching Why important – biology: curved/bent DNA important in packing into nuclei, into viruses, in regulation of transcription, various enzymes bend/twist DNA during replication, transcription, recombination technology: important for using DNA as tool to pull, twist objects; to study how various enzymes that act on DNA work; to build nanoscale objects using DNA

What we’ll cover: stretching – low force concept of entropic spring freely-jointed chain and worm-like chain models high force –> structural change in double helix B->S form, similarity to phase change methods used to study hydrodynamic drag paramagnetic beads laser traps example of how used to study mechanism of enzyme that works on DNA

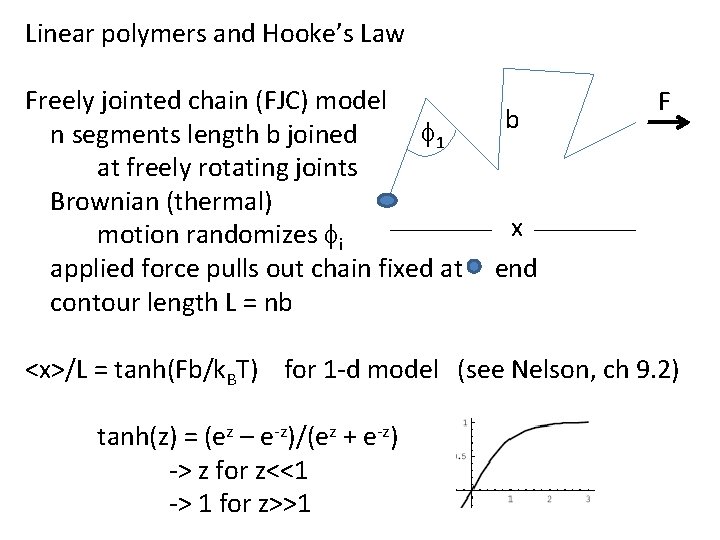
Linear polymers and Hooke’s Law Freely jointed chain (FJC) model f 1 n segments length b joined at freely rotating joints Brownian (thermal) motion randomizes fi applied force pulls out chain fixed at contour length L = nb b F x end <x>/L = tanh(Fb/k. BT) for 1 -d model (see Nelson, ch 9. 2) tanh(z) = (ez – e-z)/(ez + e-z) -> z for z<<1 -> 1 for z>>1

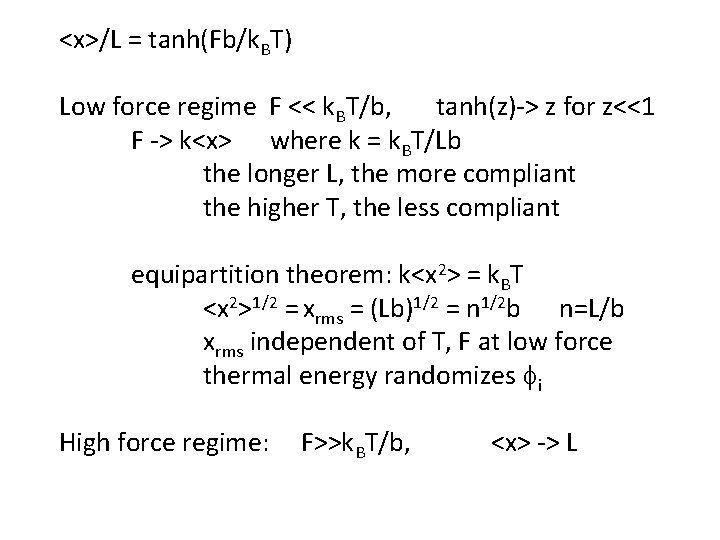
<x>/L = tanh(Fb/k. BT) Low force regime F << k. BT/b, tanh(z)-> z for z<<1 F -> k<x> where k = k. BT/Lb the longer L, the more compliant the higher T, the less compliant equipartition theorem: k<x 2> = k. BT <x 2>1/2 = xrms = (Lb)1/2 = n 1/2 b n=L/b xrms independent of T, F at low force thermal energy randomizes fi High force regime: F>>k. BT/b, <x> -> L

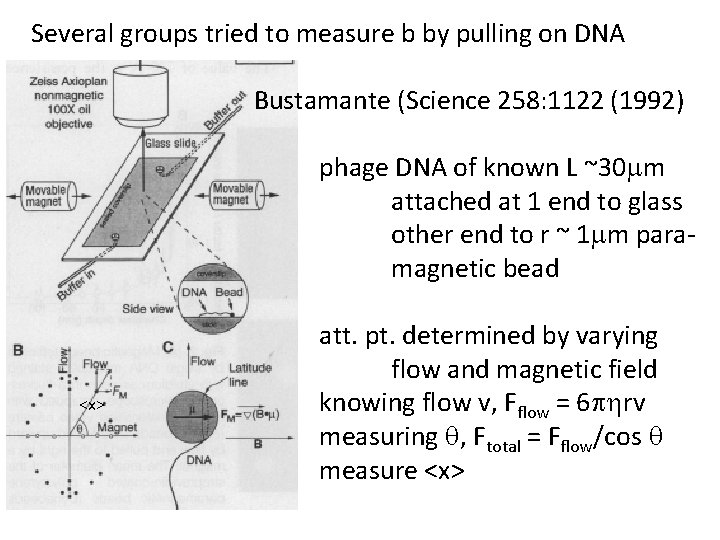
Several groups tried to measure b by pulling on DNA Bustamante (Science 258: 1122 (1992) phage DNA of known L ~30 mm attached at 1 end to glass other end to r ~ 1 mm paramagnetic bead <x> att. pt. determined by varying flow and magnetic field knowing flow v, Fflow = 6 phrv measuring q, Ftotal = Fflow/cos q measure <x>

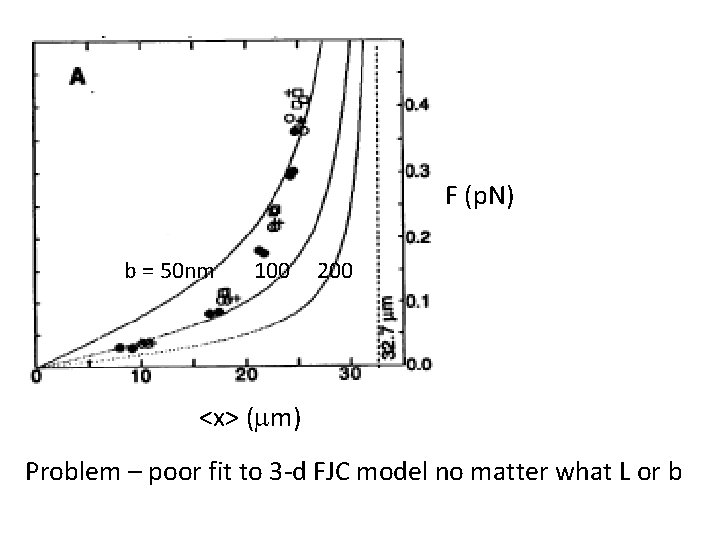
F (p. N) b = 50 nm 100 200 <x> (mm) Problem – poor fit to 3 -d FJC model no matter what L or b

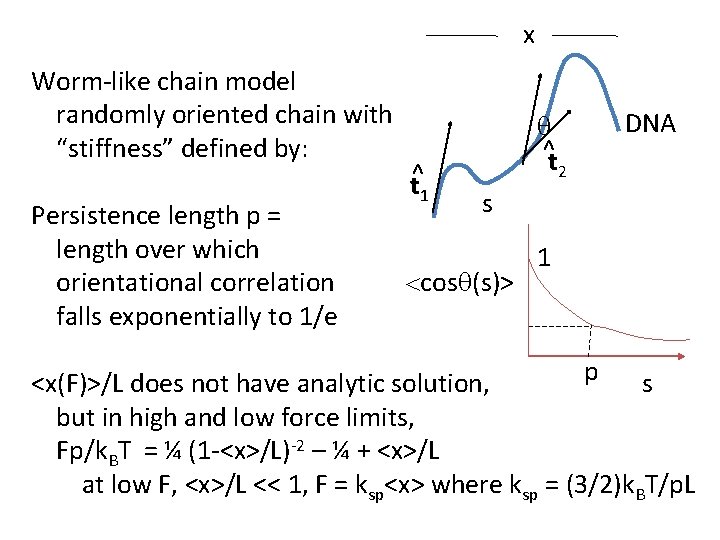
x Worm-like chain model randomly oriented chain with “stiffness” defined by: Persistence length p = length over which orientational correlation falls exponentially to 1/e t^1 q ^t 2 DNA s <cosq(s)> 1 p s <x(F)>/L does not have analytic solution, but in high and low force limits, Fp/k. BT = ¼ (1 -<x>/L)-2 – ¼ + <x>/L at low F, <x>/L << 1, F = ksp<x> where ksp = (3/2)k. BT/p. L

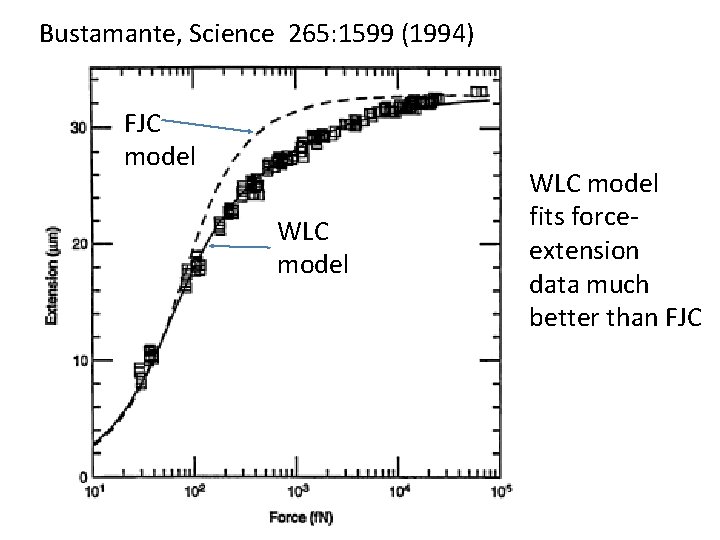
Bustamante, Science 265: 1599 (1994) FJC model WLC model fits forceextension data much better than FJC

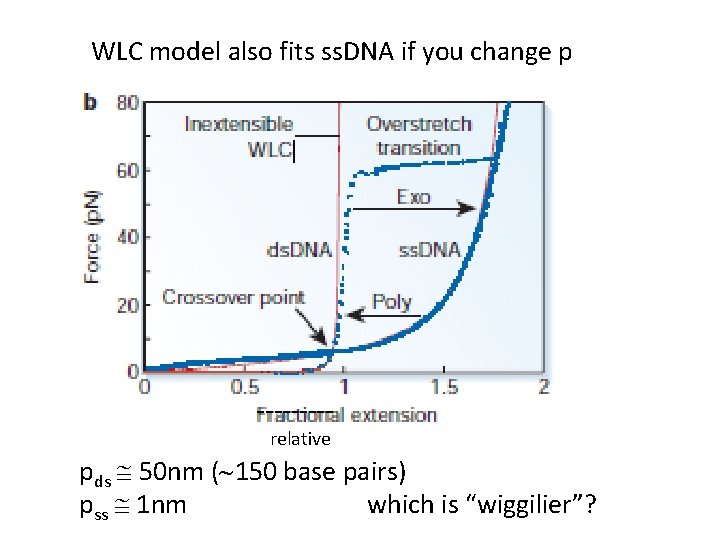
WLC model also fits ss. DNA if you change p ----relative pds @ 50 nm (~150 base pairs) pss @ 1 nm which is “wiggilier”?

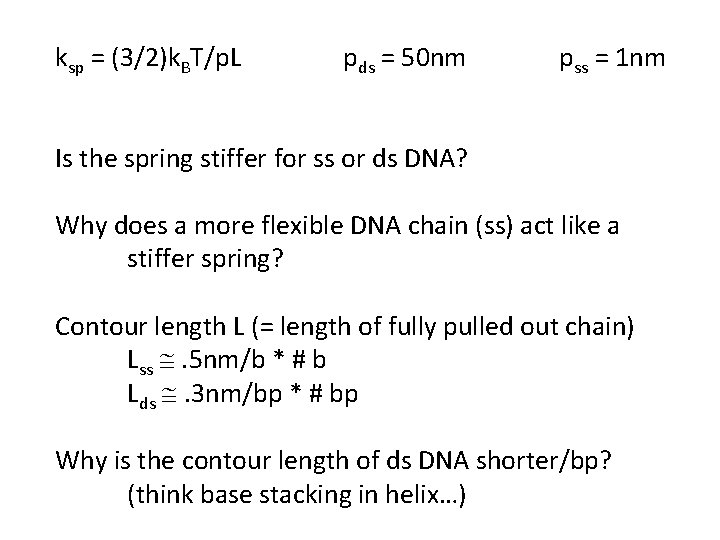
ksp = (3/2)k. BT/p. L pds = 50 nm pss = 1 nm Is the spring stiffer for ss or ds DNA? Why does a more flexible DNA chain (ss) act like a stiffer spring? Contour length L (= length of fully pulled out chain) Lss @. 5 nm/b * # b Lds @. 3 nm/bp * # bp Why is the contour length of ds DNA shorter/bp? (think base stacking in helix…)

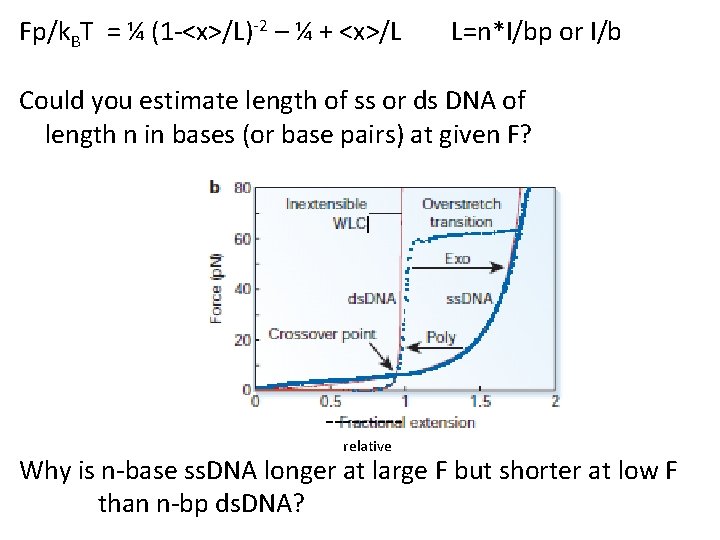
Fp/k. BT = ¼ (1 -<x>/L)-2 – ¼ + <x>/L L=n*l/bp or l/b Could you estimate length of ss or ds DNA of length n in bases (or base pairs) at given F? ----relative Why is n-base ss. DNA longer at large F but shorter at low F than n-bp ds. DNA?

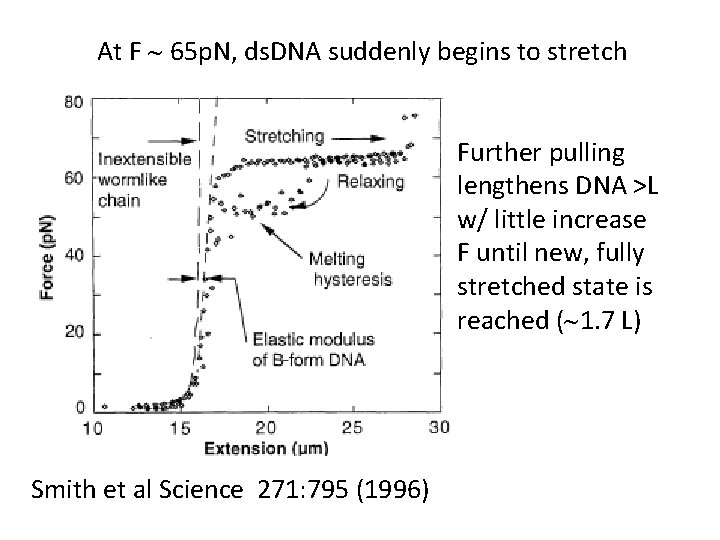
At F ~ 65 p. N, ds. DNA suddenly begins to stretch Further pulling lengthens DNA >L w/ little increase F until new, fully stretched state is reached (~1. 7 L) Smith et al Science 271: 795 (1996)

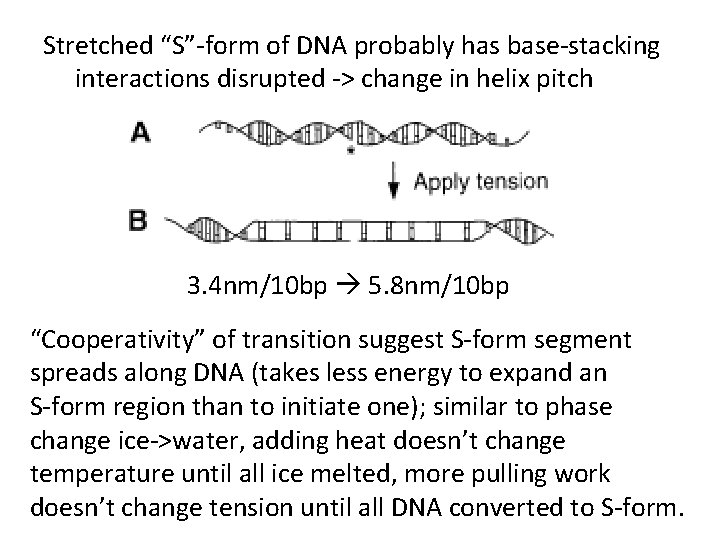
Stretched “S”-form of DNA probably has base-stacking interactions disrupted -> change in helix pitch 3. 4 nm/10 bp 5. 8 nm/10 bp “Cooperativity” of transition suggest S-form segment spreads along DNA (takes less energy to expand an S-form region than to initiate one); similar to phase change ice->water, adding heat doesn’t change temperature until all ice melted, more pulling work doesn’t change tension until all DNA converted to S-form.

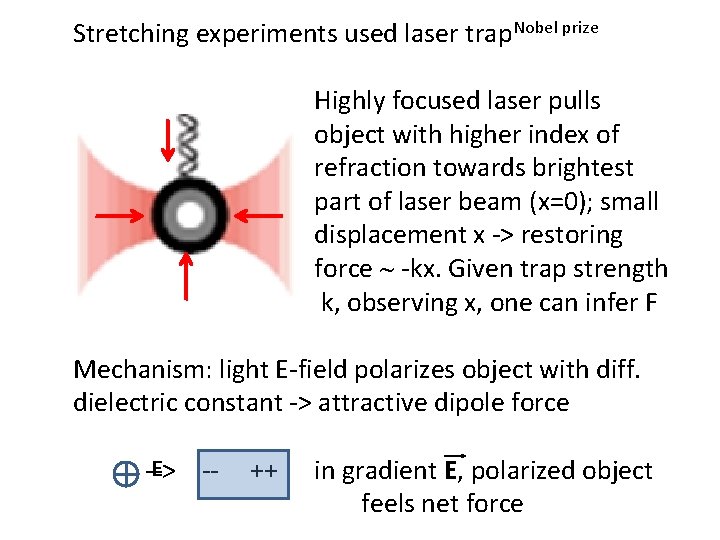
Stretching experiments used laser trap. Nobel prize Highly focused laser pulls object with higher index of refraction towards brightest part of laser beam (x=0); small displacement x -> restoring force ~ -kx. Given trap strength k, observing x, one can infer F Mechanism: light E-field polarizes object with diff. dielectric constant -> attractive dipole force E --> -- ++ in gradient E, polarized object feels net force

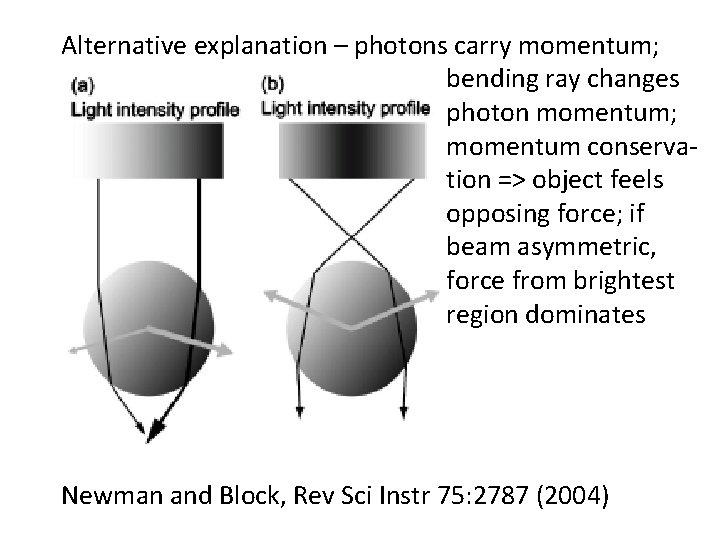
Alternative explanation – photons carry momentum; bending ray changes photon momentum; momentum conservation => object feels opposing force; if beam asymmetric, force from brightest region dominates Newman and Block, Rev Sci Instr 75: 2787 (2004)

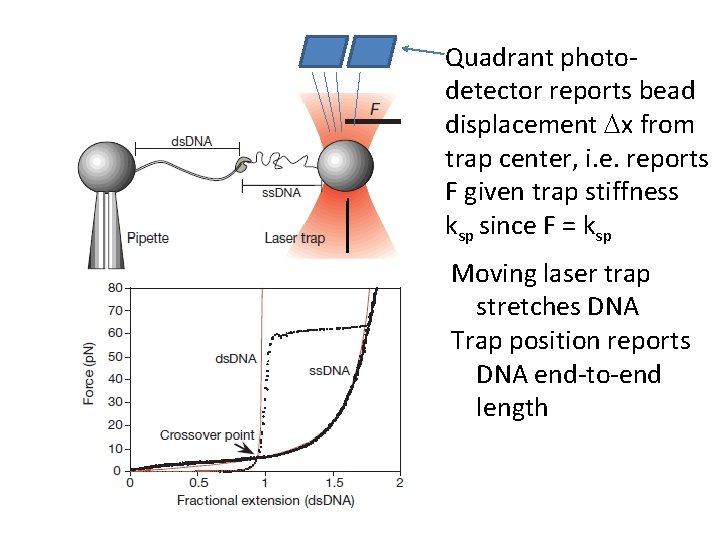
Quadrant photodetector reports bead displacement Dx from trap center, i. e. reports F given trap stiffness ksp since F = ksp Moving laser trap stretches DNA Trap position reports DNA end-to-end length

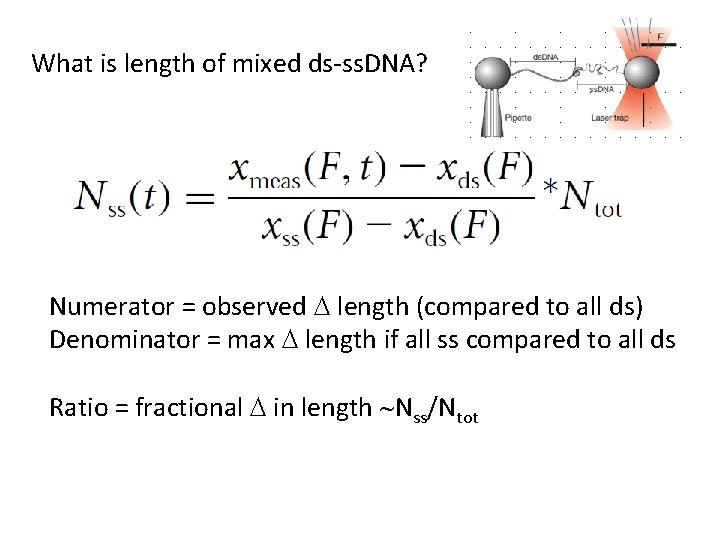
What is length of mixed ds-ss. DNA? Numerator = observed D length (compared to all ds) Denominator = max D length if all ss compared to all ds Ratio = fractional D in length ~Nss/Ntot

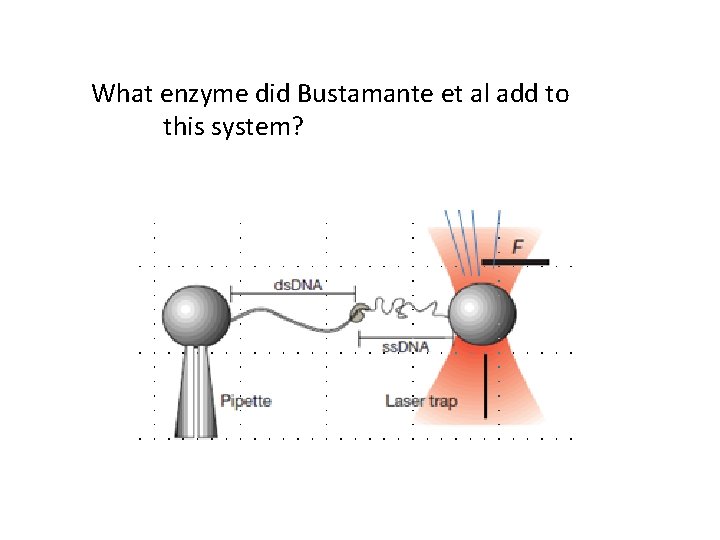
What enzyme did Bustamante et al add to this system?

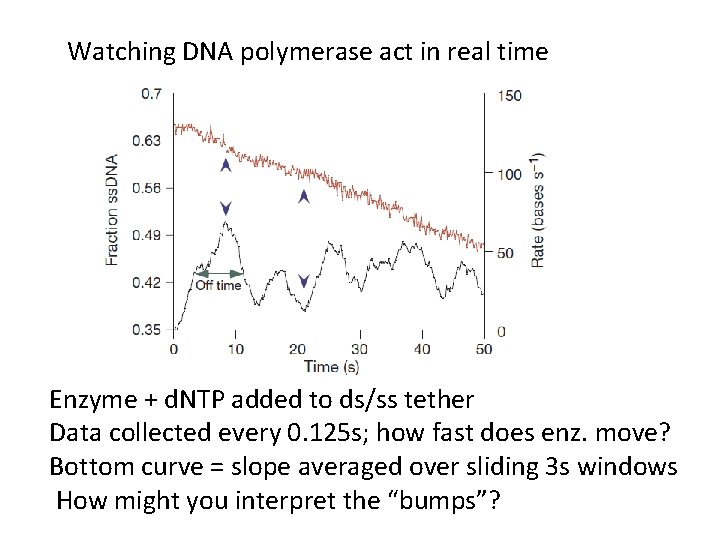
Watching DNA polymerase act in real time Enzyme + d. NTP added to ds/ss tether Data collected every 0. 125 s; how fast does enz. move? Bottom curve = slope averaged over sliding 3 s windows How might you interpret the “bumps”?

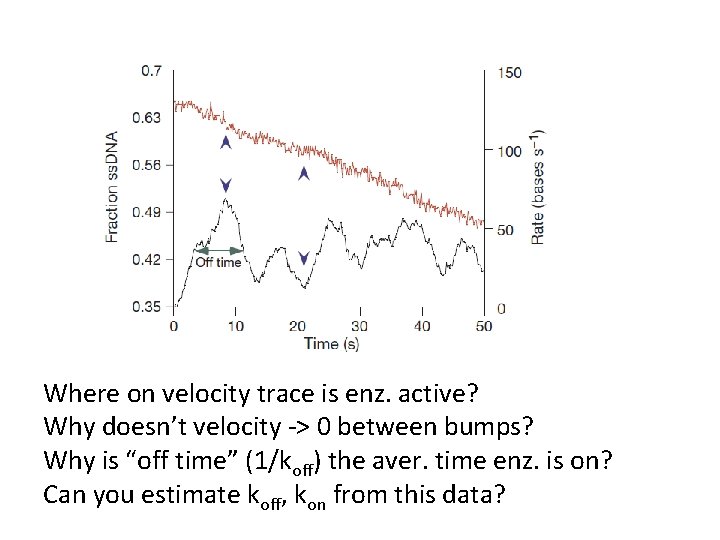
Where on velocity trace is enz. active? Why doesn’t velocity -> 0 between bumps? Why is “off time” (1/koff) the aver. time enz. is on? Can you estimate koff, kon from this data?

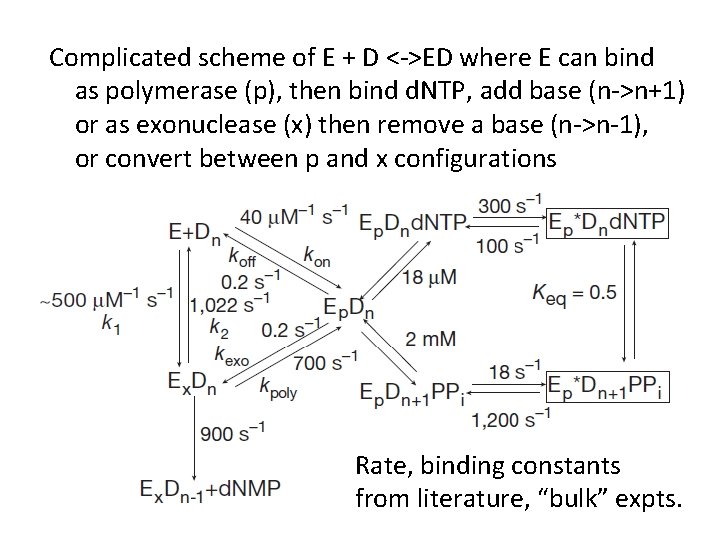
Complicated scheme of E + D <->ED where E can bind as polymerase (p), then bind d. NTP, add base (n->n+1) or as exonuclease (x) then remove a base (n->n-1), or convert between p and x configurations Rate, binding constants from literature, “bulk” expts.

You could compare your single-molecule kon, koff to data from bulk expts; this might strengthen your interpretation but does not advance the field What is biological role of exonuclease function? What happens to misincorporation rate if you mutate (eliminate) exo function?

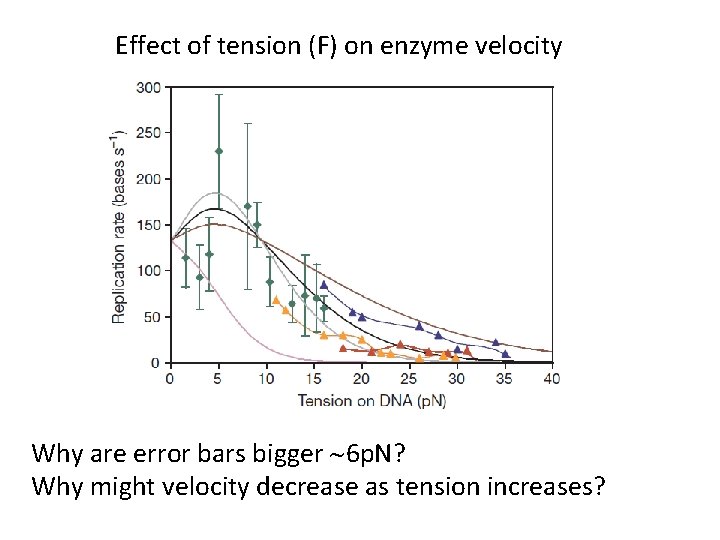
Effect of tension (F) on enzyme velocity Why are error bars bigger ~6 p. N? Why might velocity decrease as tension increases?

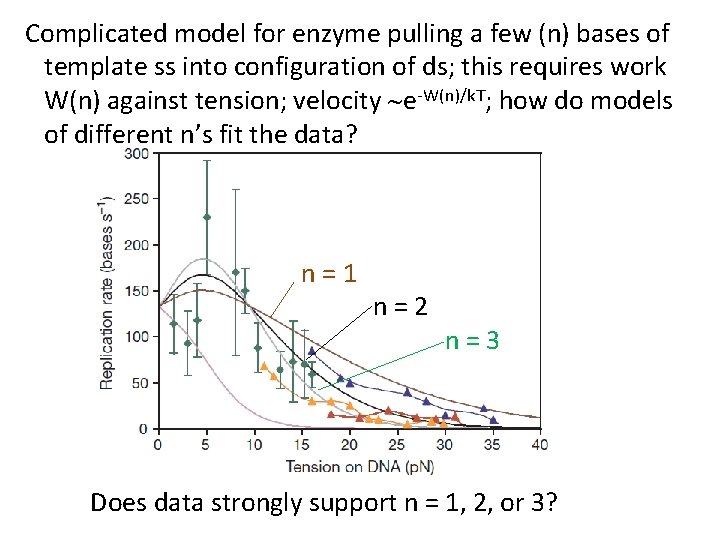
Complicated model for enzyme pulling a few (n) bases of template ss into configuration of ds; this requires work W(n) against tension; velocity ~e-W(n)/k. T; how do models of different n’s fit the data? n=1 n=2 n=3 Does data strongly support n = 1, 2, or 3?

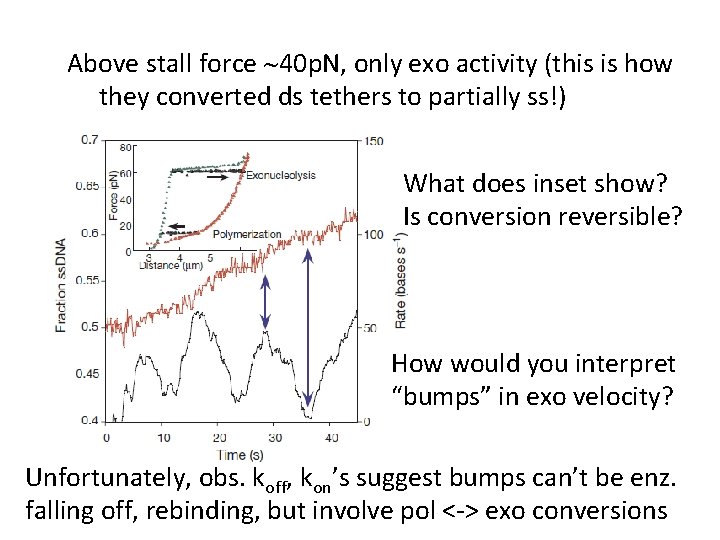
Above stall force ~40 p. N, only exo activity (this is how they converted ds tethers to partially ss!) What does inset show? Is conversion reversible? How would you interpret “bumps” in exo velocity? Unfortunately, obs. koff, kon’s suggest bumps can’t be enz. falling off, rebinding, but involve pol <-> exo conversions

What can single-molecule expts. show that would be very hard to learn from bulk expts. ? Are enzyme molecules heterogeneous or all the same? Is enzyme rate sequence-dependent? Is enzyme rate slowed by tension? This could inform detailed models of how enzyme works What makes enzyme interconvert between pol and exo conformations?

Summary laser traps/magnets/tethered bead expt’l. system: allow application of p. N forces measurement of p. N forces and DNA/RNA lengths with near nm precision WLC model predicts DNA mechanical properties accurately (extension as function of force, twist and buckling as function of torque) Clever experimental systems -> real-time observation of single enzymes/assemblies at work, potentially elucidating mechanistic details

Lots of other examples of single-molecule studies: RNA polymerases that partially melt ds. DNA and make RNA copies Motors that pack DNA into virus particles Helicases that unwind ds DNA/RNA Topoisomerases that nick, religate DNA, relieving torsional strain and topological entanglement Ribosomes that copy RNA into protein

These studies combine nano-scale biology and engineering -> new discipline For now, mostly research applications… Understanding nanoscale biosystems provide insight, tools potentially applicable to non-biological nanosystems

Example – experimental test of basic physics prediction of relation between work W done on non-equilibrium system and free energy change DG at equil. W > DG (due to dissipation) classical eqn <e-W/k. T> = e. DG Jarzynski prediction 1999 slow W = area betw nfold curves efold Science vol 296 fast p 1832, 2002

Next week – DNA sequencing why the interest first “next generation” method Homework problems on DNA mechanics Midterm due by end of weekend
 Rosalind frankilin
Rosalind frankilin Pictures
Pictures Why are colligative properties important
Why are colligative properties important Function of dna polymerase 3
Function of dna polymerase 3 Bioflix activity dna replication nucleotide pairing
Bioflix activity dna replication nucleotide pairing Coding dna and non coding dna
Coding dna and non coding dna The principal enzyme involved in dna replication is
The principal enzyme involved in dna replication is Dna and genes chapter 11
Dna and genes chapter 11 Actual mechanical advantage vs ideal mechanical advantage
Actual mechanical advantage vs ideal mechanical advantage Dont ask why why why
Dont ask why why why Example of a news story
Example of a news story Inverted pyramid in news writing
Inverted pyramid in news writing Least important to most important
Least important to most important Ballistic stretch definition
Ballistic stretch definition Phil wharton active isolated stretching
Phil wharton active isolated stretching Stretching spring
Stretching spring Stretching simmetrico e asimmetrico
Stretching simmetrico e asimmetrico Pnf stretching
Pnf stretching Bts pnf
Bts pnf Accessory joint motion
Accessory joint motion Lajin stretching
Lajin stretching Stretching and bending vibrations in ir spectroscopy
Stretching and bending vibrations in ir spectroscopy Stretching and shrinking math book
Stretching and shrinking math book Cold stretching cryogenic vessel
Cold stretching cryogenic vessel Stretching and bending vibrations in ir spectroscopy
Stretching and bending vibrations in ir spectroscopy Hydraulic training pdhpe
Hydraulic training pdhpe Hooke's law set up
Hooke's law set up Types of flexibility training
Types of flexibility training Flexibility objective
Flexibility objective Types of flexibility
Types of flexibility Ballistic stretching
Ballistic stretching Force extension graphs
Force extension graphs