Measuring Reaction Rates Continuous monitoring polarimetry spectrophotometry total
![First Order Reaction ln[A]0 slo pe ln[A] =− k time Tro, Chemistry: A Molecular First Order Reaction ln[A]0 slo pe ln[A] =− k time Tro, Chemistry: A Molecular](https://slidetodoc.com/presentation_image_h2/043224e4a812564b28e4a09d4484f4b0/image-11.jpg)



![Summary: First Order Reactions • Rate law: rate = k[A] • Integrated rate law: Summary: First Order Reactions • Rate law: rate = k[A] • Integrated rate law:](https://slidetodoc.com/presentation_image_h2/043224e4a812564b28e4a09d4484f4b0/image-18.jpg)

![p o l s 1/[A] k = e l/[A]0 time Tro, Chemistry: A Molecular p o l s 1/[A] k = e l/[A]0 time Tro, Chemistry: A Molecular](https://slidetodoc.com/presentation_image_h2/043224e4a812564b28e4a09d4484f4b0/image-20.jpg)

![Summary: Second Order Reactions • Rate law: rate = k[A]2 • Integrated rate law: Summary: Second Order Reactions • Rate law: rate = k[A]2 • Integrated rate law:](https://slidetodoc.com/presentation_image_h2/043224e4a812564b28e4a09d4484f4b0/image-23.jpg)
![Zero Order Reactions • Rate law: rate = k[A]0 = k • • üconstant Zero Order Reactions • Rate law: rate = k[A]0 = k • • üconstant](https://slidetodoc.com/presentation_image_h2/043224e4a812564b28e4a09d4484f4b0/image-24.jpg)
- Slides: 24

Measuring Reaction Rates • Continuous monitoring ü polarimetry ü spectrophotometry ü total pressure • Taking aliquots ü gas chromatography ü titration for one of the components ü gravimetric analysis Tro, Chemistry: A Molecular Approach

The Rate Law • The Rate Law of a reaction is the mathematical relationship • between the rate of the reaction and the concentrations of the reactants The rate of a reaction is directly proportional to the concentration of each reactant raised to a power • For the reaction a. A + b. B products the rate law would have the form given below ü n and m are called the orders for each reactant ü k is called the rate constant Tro, Chemistry: A Molecular Approach

Reaction Order • The exponent on each reactant in the rate law is called • • the order with respect to that reactant The sum of the exponents on the reactants is called the order of the reaction The rate law for the reaction: 2 NO(g) + O 2(g) 2 NO 2(g) is Rate = k[NO]2[O 2] • The reaction is second order with respect to [NO], first order with respect to [O 2], and third order overall. Tro, Chemistry: A Molecular Approach

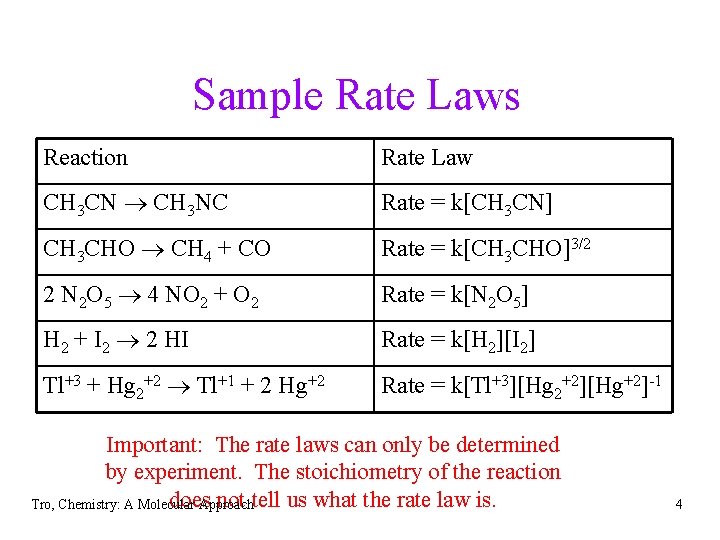
Sample Rate Laws Reaction Rate Law CH 3 CN CH 3 NC Rate = k[CH 3 CN] CH 3 CHO CH 4 + CO Rate = k[CH 3 CHO]3/2 2 N 2 O 5 4 NO 2 + O 2 Rate = k[N 2 O 5] H 2 + I 2 2 HI Rate = k[H 2][I 2] Tl+3 + Hg 2+2 Tl+1 + 2 Hg+2 Rate = k[Tl+3][Hg 2+2][Hg+2]-1 Important: The rate laws can only be determined by experiment. The stoichiometry of the reaction does not tell us what the rate law is. Tro, Chemistry: A Molecular Approach 4

Why is the Rate Law Important? • The concentrations of reactants and products can • be predicted for any time throughout the reaction It can be used to propose reaction mechanisms that give insights into what is happening in the reaction on the molecular level. Tro, Chemistry: A Molecular Approach

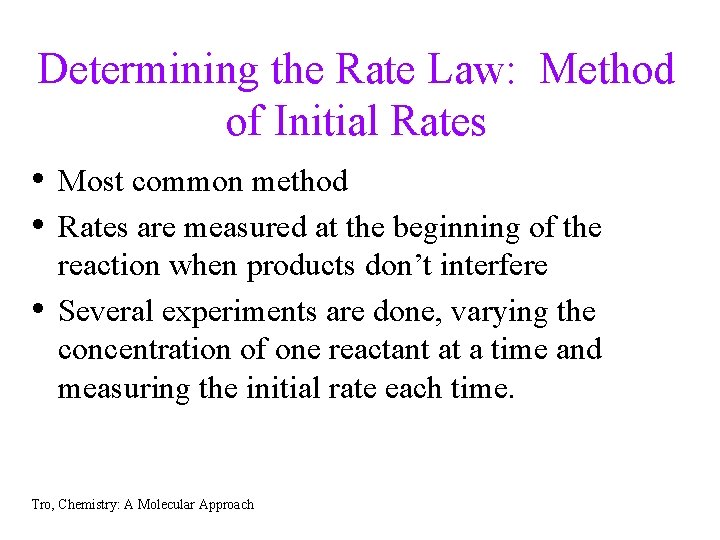
Determining the Rate Law: Method of Initial Rates • Most common method • Rates are measured at the beginning of the • reaction when products don’t interfere Several experiments are done, varying the concentration of one reactant at a time and measuring the initial rate each time. Tro, Chemistry: A Molecular Approach

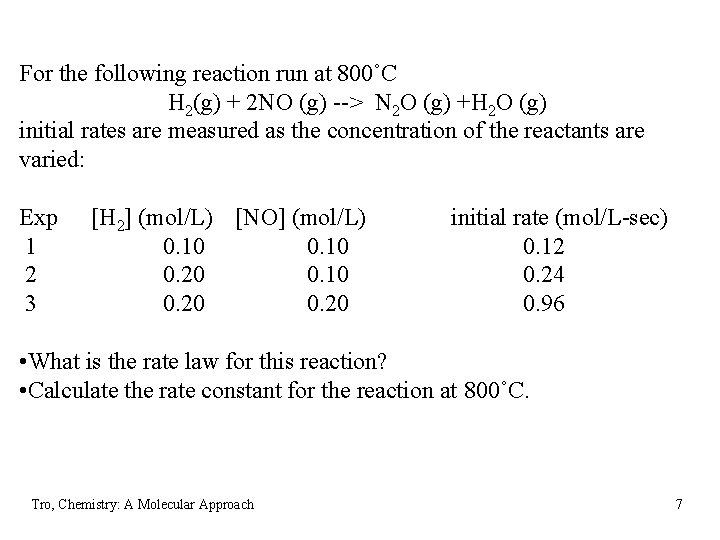
For the following reaction run at 800˚C H 2(g) + 2 NO (g) --> N 2 O (g) +H 2 O (g) initial rates are measured as the concentration of the reactants are varied: Exp 1 2 3 [H 2] (mol/L) [NO] (mol/L) 0. 10 0. 20 initial rate (mol/L-sec) 0. 12 0. 24 0. 96 • What is the rate law for this reaction? • Calculate the rate constant for the reaction at 800˚C. Tro, Chemistry: A Molecular Approach 7

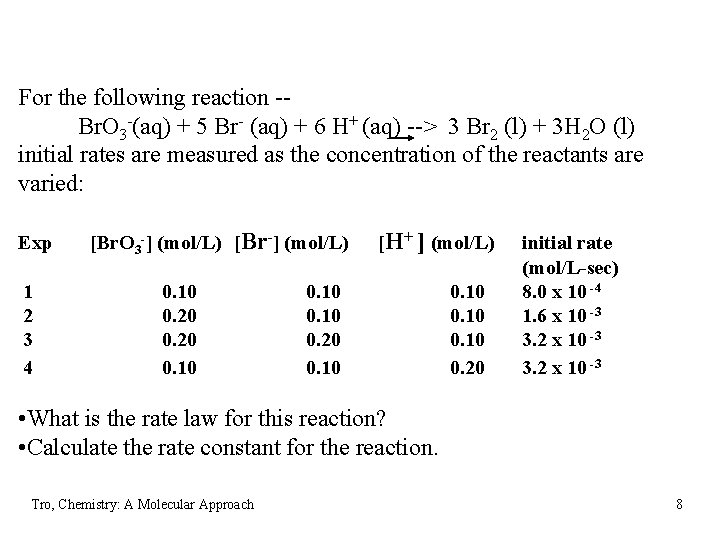
For the following reaction -Br. O 3 -(aq) + 5 Br- (aq) + 6 H+ (aq) --> 3 Br 2 (l) + 3 H 2 O (l) initial rates are measured as the concentration of the reactants are varied: Exp 1 2 3 4 [Br. O 3 -] (mol/L) [Br-] (mol/L) 0. 10 0. 20 0. 10 [H+ ] (mol/L) 0. 10 0. 20 initial rate (mol/L-sec) 8. 0 x 10 -4 1. 6 x 10 -3 3. 2 x 10 -3 • What is the rate law for this reaction? • Calculate the rate constant for the reaction. Tro, Chemistry: A Molecular Approach 8

Integrated Rate Laws: Predicting Concentrations as a Function of Time • We are going to discuss integrated rate laws for reactions that are zero, first and second order in one reactant only. üRate = k[A]0 üRate = k[A]2 “zeroth” order first order second order • Other rate laws are too complicated mathematically Tro, Chemistry: A Molecular Approach

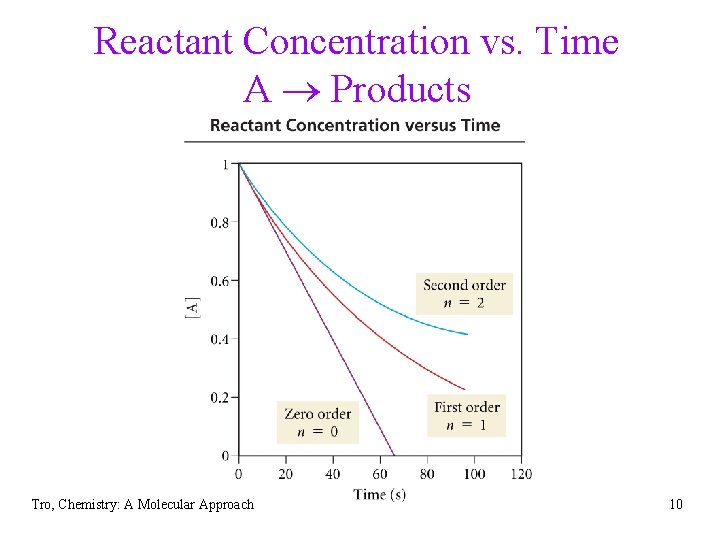
Reactant Concentration vs. Time A Products Tro, Chemistry: A Molecular Approach 10
![First Order Reaction lnA0 slo pe lnA k time Tro Chemistry A Molecular First Order Reaction ln[A]0 slo pe ln[A] =− k time Tro, Chemistry: A Molecular](https://slidetodoc.com/presentation_image_h2/043224e4a812564b28e4a09d4484f4b0/image-11.jpg)
First Order Reaction ln[A]0 slo pe ln[A] =− k time Tro, Chemistry: A Molecular Approach 11

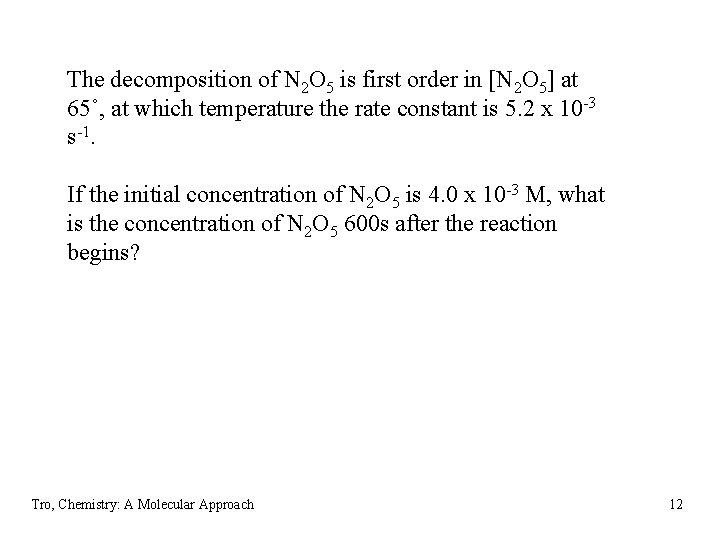
The decomposition of N 2 O 5 is first order in [N 2 O 5] at 65˚, at which temperature the rate constant is 5. 2 x 10 -3 s-1. If the initial concentration of N 2 O 5 is 4. 0 x 10 -3 M, what is the concentration of N 2 O 5 600 s after the reaction begins? Tro, Chemistry: A Molecular Approach 12

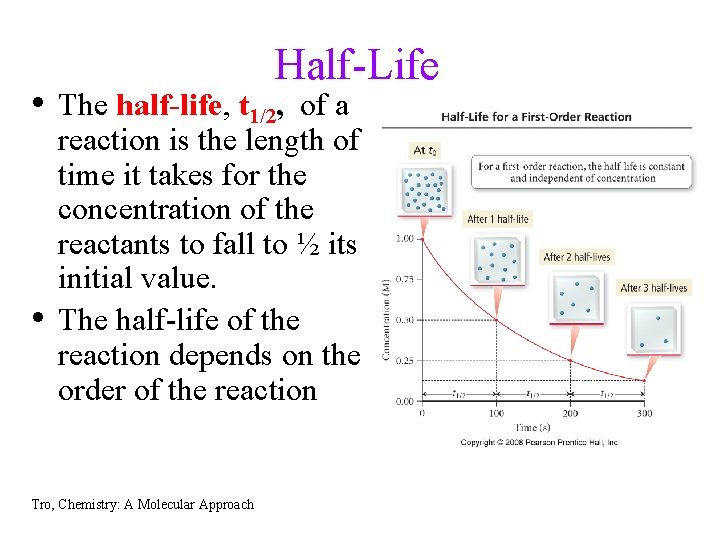
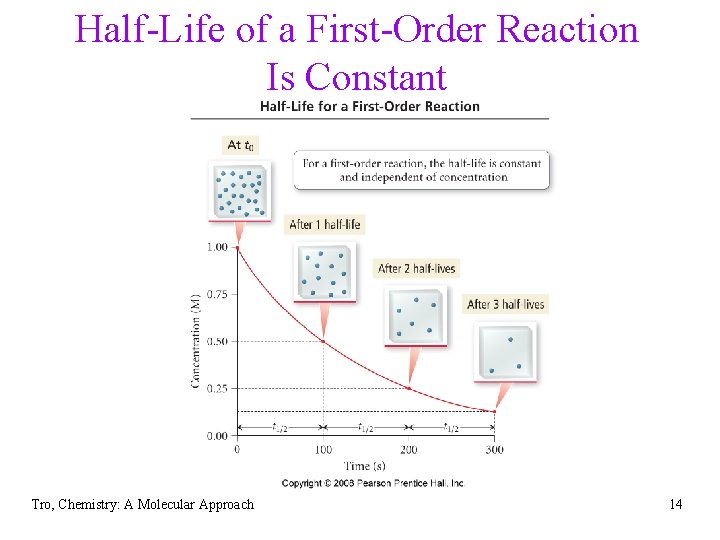
Half-Life • The half-life, t 1/2, of a • reaction is the length of time it takes for the concentration of the reactants to fall to ½ its initial value. The half-life of the reaction depends on the order of the reaction Tro, Chemistry: A Molecular Approach

Half-Life of a First-Order Reaction Is Constant Tro, Chemistry: A Molecular Approach 14

Half-life for a First Order Reaction Tro, Chemistry: A Molecular Approach 15

In the N 2 O 5 decomposition, after what time will half of the reactants decompose at 65˚C? Tro, Chemistry: A Molecular Approach 16

A certain first order reaction has a half life of 20. 0 minutes. 1. Calculate the rate constant for this reaction. 2. How much time is required for this reaction to be 75% complete? Tro, Chemistry: A Molecular Approach 17
![Summary First Order Reactions Rate law rate kA Integrated rate law Summary: First Order Reactions • Rate law: rate = k[A] • Integrated rate law:](https://slidetodoc.com/presentation_image_h2/043224e4a812564b28e4a09d4484f4b0/image-18.jpg)
Summary: First Order Reactions • Rate law: rate = k[A] • Integrated rate law: ln[A] = -kt + ln[A]0 • Graph: ln[A] vs. time gives straight line ü slope = -k and y-intercept = ln[A]0 üused to determine the rate constant • Half-life üt½ = 0. 693/k üThe half-life of a first order reaction is constant • Units for k: sec-1 Tro, Chemistry: A Molecular Approach

Second Order Reaction Tro, Chemistry: A Molecular Approach 19
![p o l s 1A k e lA0 time Tro Chemistry A Molecular p o l s 1/[A] k = e l/[A]0 time Tro, Chemistry: A Molecular](https://slidetodoc.com/presentation_image_h2/043224e4a812564b28e4a09d4484f4b0/image-20.jpg)
p o l s 1/[A] k = e l/[A]0 time Tro, Chemistry: A Molecular Approach 20

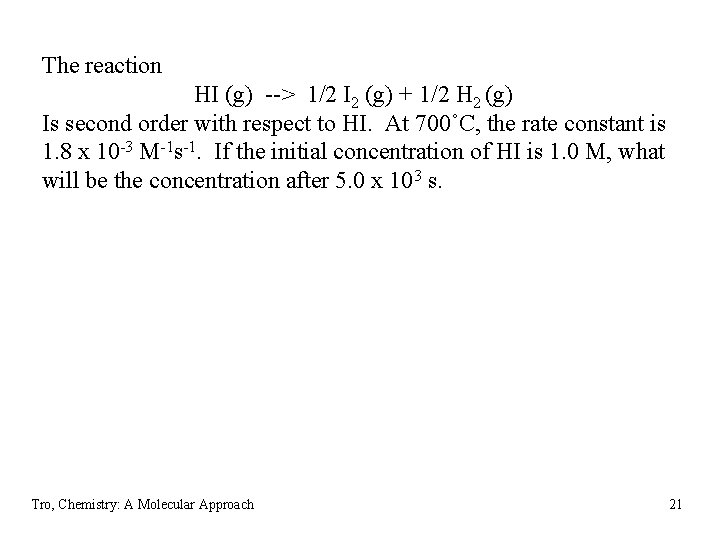
The reaction HI (g) --> 1/2 I 2 (g) + 1/2 H 2 (g) Is second order with respect to HI. At 700˚C, the rate constant is 1. 8 x 10 -3 M-1 s-1. If the initial concentration of HI is 1. 0 M, what will be the concentration after 5. 0 x 103 s. Tro, Chemistry: A Molecular Approach 21

The half-life of a second order reaction depends on the concentration of the reactant or reactants. Tro, Chemistry: A Molecular Approach 22
![Summary Second Order Reactions Rate law rate kA2 Integrated rate law Summary: Second Order Reactions • Rate law: rate = k[A]2 • Integrated rate law:](https://slidetodoc.com/presentation_image_h2/043224e4a812564b28e4a09d4484f4b0/image-23.jpg)
Summary: Second Order Reactions • Rate law: rate = k[A]2 • Integrated rate law: 1/[A] = kt + 1/[A]0 • oooo 1/[A] vs. time gives straight line ü slope = k and y-intercept = 1/[A]0 üused to determine the rate constant • Half life: t½ = 1/(k[A 0]) • Units for k: k = M-1∙sec-1 Tro, Chemistry: A Molecular Approach
![Zero Order Reactions Rate law rate kA0 k üconstant Zero Order Reactions • Rate law: rate = k[A]0 = k • • üconstant](https://slidetodoc.com/presentation_image_h2/043224e4a812564b28e4a09d4484f4b0/image-24.jpg)
Zero Order Reactions • Rate law: rate = k[A]0 = k • • üconstant rate reactions Integrated rate law: [A] = -kt + [A]0 Graph: [A] vs. time is straight line with slope = -k and y-intercept = [A]0 t ½ = [A 0]/2 k Units: if Rate = M/sec, k = M/sec [A]0 [A] Tro, Chemistry: A Molecular Approach slo pe time =- k