MEASURING RATE CONSTANTS FOR REACTIONS OF THE SIMPLEST



![Increase [HFA] LIF signals for OH as a function of time for three different Increase [HFA] LIF signals for OH as a function of time for three different](https://slidetodoc.com/presentation_image/086659acb20c6b84bf8cd4224ba0d3ff/image-11.jpg)
![Summary Ø [CH 2 OO] = const×[OH] use OH time profile to represent the Summary Ø [CH 2 OO] = const×[OH] use OH time profile to represent the](https://slidetodoc.com/presentation_image/086659acb20c6b84bf8cd4224ba0d3ff/image-13.jpg)


- Slides: 16

MEASURING RATE CONSTANTS FOR REACTIONS OF THE SIMPLEST CRIEGEE INTERMEDIATE CH 2 OO BY MONITORING THE OH RADICAL YINGDI LIU, KYLE D BAYES AND STANLEY P. SANDER JET PROPULSION LABORATORY, CALIFORNIA INSTITUTE OF TECHNOLOGY, CA 1

2

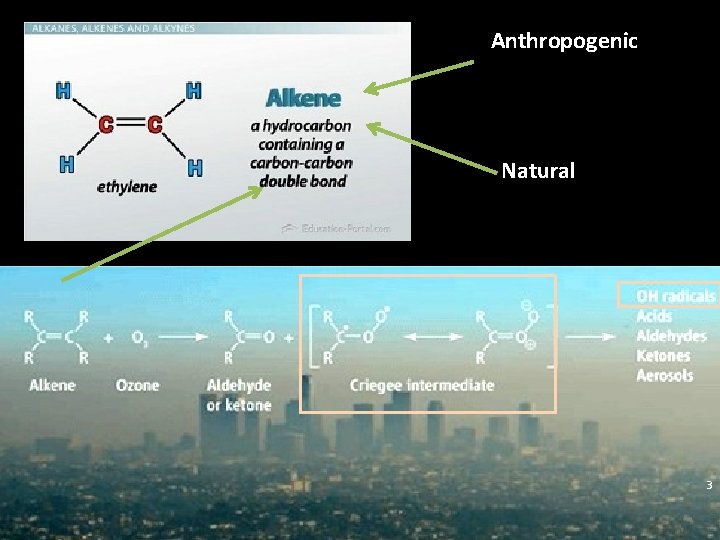
Anthropogenic Natural 3

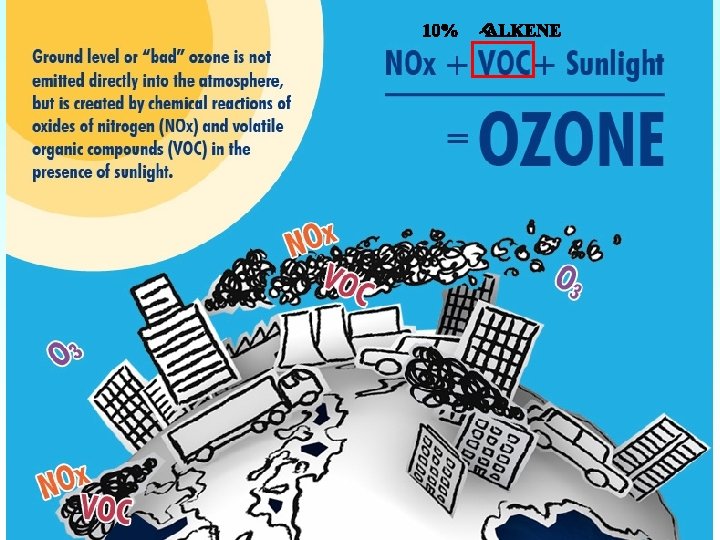
Ozone and alkene reaction Ø Production of OH radical Ø An important source of HOx Ø The observed yields: 10% - 100%. Ø Generate Criegee intermediate (CI) Ø CI reacts with many important molecules in the atmosphere Ø A potential Secondary organic aerosol precursor Ø OH production mechanism is not completely established Ø Lack of CI kinetics information 4

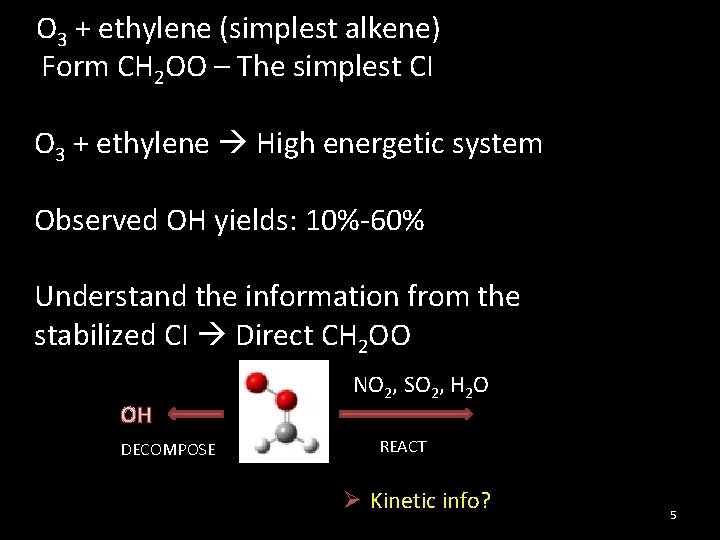
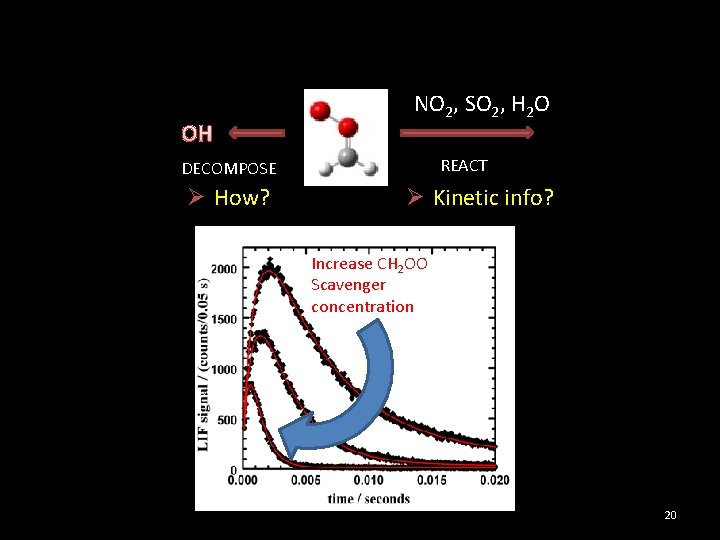
O 3 + ethylene (simplest alkene) Form CH 2 OO – The simplest CI O 3 + ethylene High energetic system Observed OH yields: 10%-60% Understand the information from the stabilized CI Direct CH 2 OO OH DECOMPOSE NO 2, SO 2, H 2 O REACT Ø Kinetic info? 5

Motivation ØGenerate CH 2 OO directly without O 3 + ethylene reaction “direct”CH 2 OO. ØDirect monitoring OH formation from “direct”CH 2 OO. ØIndirectly measure the kinetics info of CH 2 OO 6

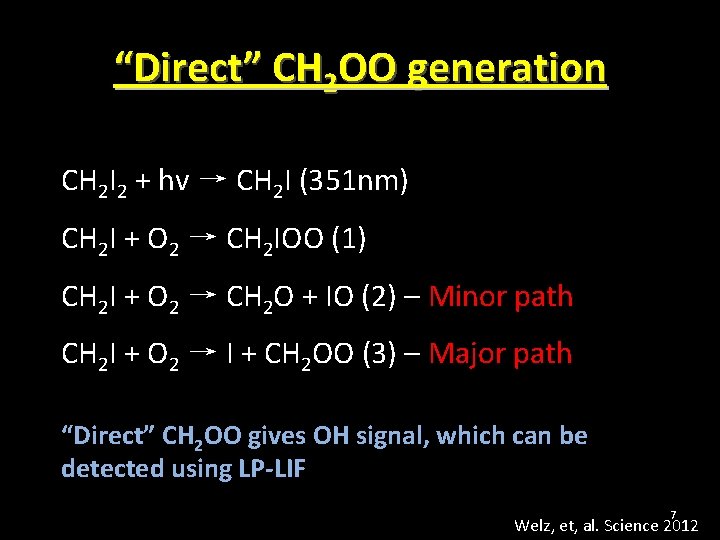
“Direct” CH 2 OO generation CH 2 I 2 + hv → CH 2 I (351 nm) CH 2 I + O 2 → CH 2 IOO (1) CH 2 I + O 2 → CH 2 O + IO (2) – Minor path CH 2 I + O 2 → I + CH 2 OO (3) – Major path “Direct” CH 2 OO gives OH signal, which can be detected using LP-LIF 7 Welz, et, al. Science 2012

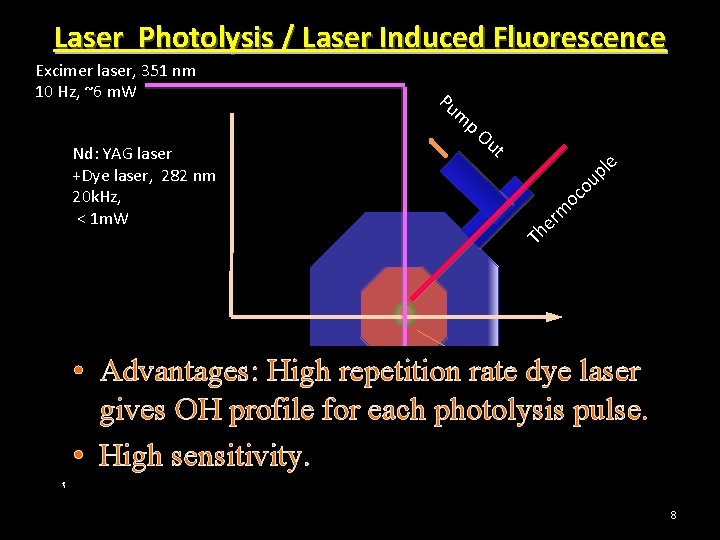
Laser Photolysis / Laser Induced Fluorescence Excimer laser, 351 nm 10 Hz, ~6 m. W Pu m p Nd: YAG laser +Dye laser, 282 nm 20 k. Hz, < 1 m. W Ou t e l p u o oc m T r e h 2 Ar + +O + CH 2 HF 2 I A 2 or SO • Advantages: High repetition rate dye laser gives OH profile for each photolysis pulse. Photomultiplier Tube + Photon counting • High sensitivity. OH Signal 8

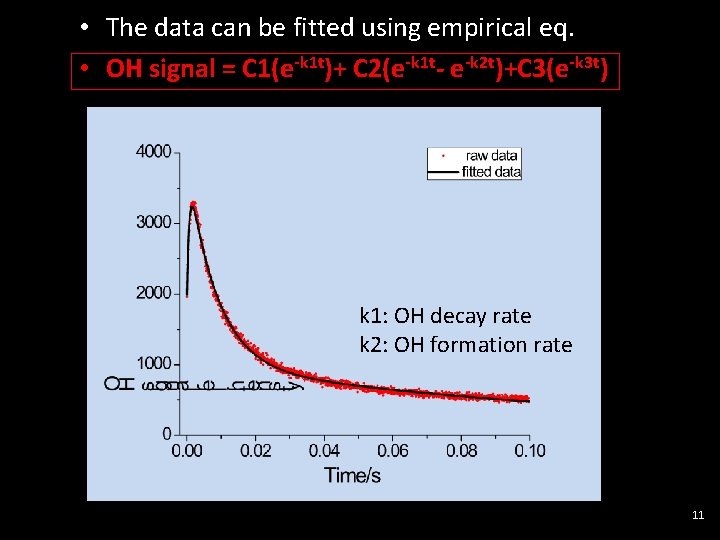
• The data can be fitted using empirical eq. • OH signal = C 1(e-k 1 t)+ C 2(e-k 1 t- e-k 2 t)+C 3(e-k 3 t) k 1: OH decay rate k 2: OH formation rate 11

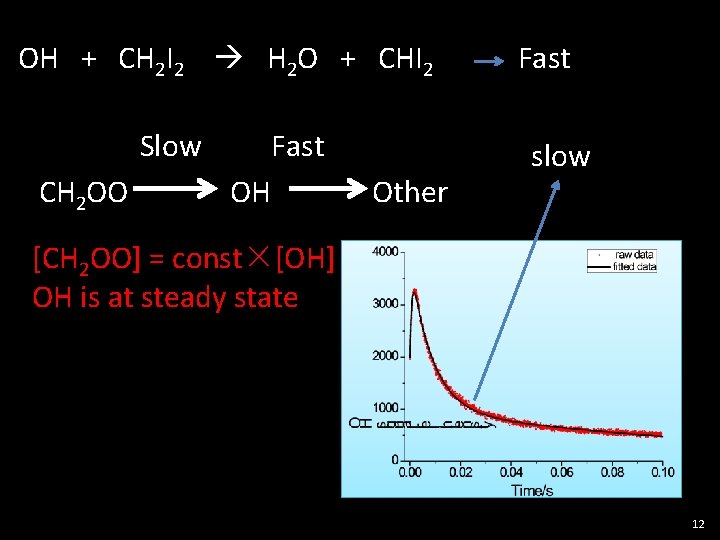
OH + CH 2 I 2 H 2 O + CHI 2 Slow CH 2 OO Fast OH Other Fast slow [CH 2 OO] = const×[OH] OH is at steady state 12
![Increase HFA LIF signals for OH as a function of time for three different Increase [HFA] LIF signals for OH as a function of time for three different](https://slidetodoc.com/presentation_image/086659acb20c6b84bf8cd4224ba0d3ff/image-11.jpg)
Increase [HFA] LIF signals for OH as a function of time for three different concentrations of HFA = hexafluoroacetone HFA does not react with OH 15 The fitted decay constant K 1 for different concentrations of HFA. Slope = rate constant of CH 2 OO with HFA. (3. 33 ± 0. 27) x 10 -11 cm 3 molecule-1 s-1 CH 2 OO + HFA Adduct

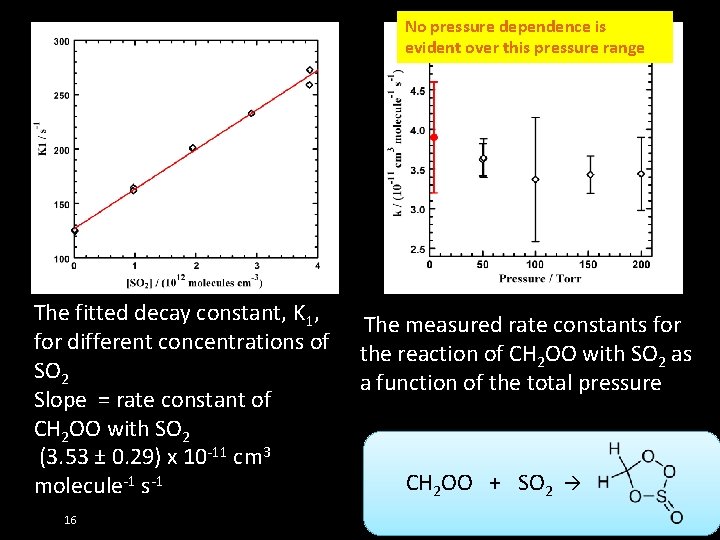
No pressure dependence is evident over this pressure range The fitted decay constant, K 1, for different concentrations of SO 2 Slope = rate constant of CH 2 OO with SO 2 (3. 53 ± 0. 29) x 10 -11 cm 3 molecule-1 s-1 16 The measured rate constants for the reaction of CH 2 OO with SO 2 as a function of the total pressure CH 2 OO + SO 2
![Summary Ø CH 2 OO constOH use OH time profile to represent the Summary Ø [CH 2 OO] = const×[OH] use OH time profile to represent the](https://slidetodoc.com/presentation_image/086659acb20c6b84bf8cd4224ba0d3ff/image-13.jpg)
Summary Ø [CH 2 OO] = const×[OH] use OH time profile to represent the CH 2 OO time profile. ØBimolecular reaction rate constants of CH 2 OO with different molecules can be obtained. ØRate constants of CH 2 OO + HFA and CH 2 OO + SO 2 are obtained and the results agree with the literature. ØRate constant of CH 2 OO + SO 2 is pressure independent. 17

Acknowledgement Dr. Stanley Sander Dr. Kyle Bayes Thank you~ 18

Why signal increases in O 3 study? • O 3 + hv(282 nm) O 1 D + O 2 • O 1 D + CH 2 I 2 CHI 2 + OH 19

OH NO 2, SO 2, H 2 O REACT DECOMPOSE Ø How? Ø Kinetic info? Increase CH 2 OO Scavenger concentration 20
 Types of reactions
Types of reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet How to write redox half reactions
How to write redox half reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Factors that affect the rate of reactions
Factors that affect the rate of reactions Measurement of interest
Measurement of interest Measuring exposure to exchange rate fluctuations
Measuring exposure to exchange rate fluctuations Measuring exposure to exchange rate fluctuations
Measuring exposure to exchange rate fluctuations Constants in scientific method
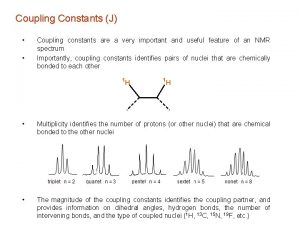
Constants in scientific method Geminal and vicinal coupling constants
Geminal and vicinal coupling constants Geminal coupling
Geminal coupling C chart six sigma
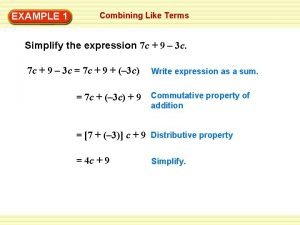
C chart six sigma Simplify the expression. (7 – c )(–1)
Simplify the expression. (7 – c )(–1) Equiibrium constant
Equiibrium constant What is an integer constant
What is an integer constant Stress strain curve toughness
Stress strain curve toughness