Measurements Number followed by a Unit SI Units
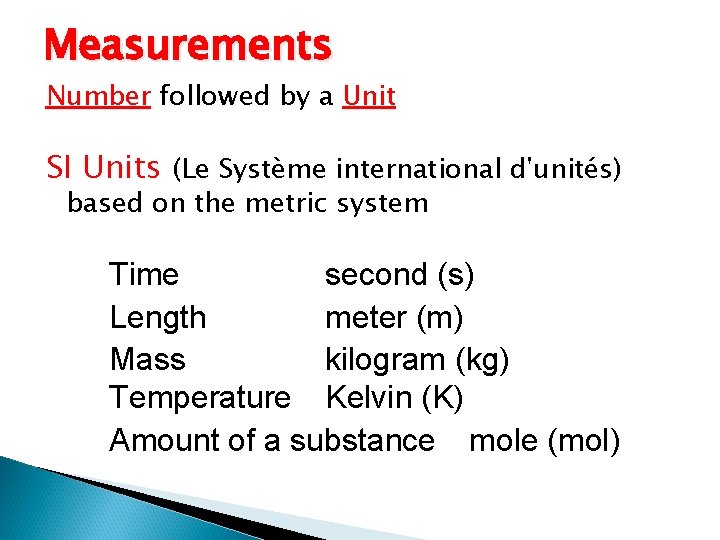
Measurements Number followed by a Unit SI Units (Le Système international d'unités) based on the metric system Time second (s) Length meter (m) Mass kilogram (kg) Temperature Kelvin (K) Amount of a substance mole (mol)

Derived Units: a combination of 2 or more base units Area Speed Volume Density (length x length) (length per time) (length x length) (mass per volume)
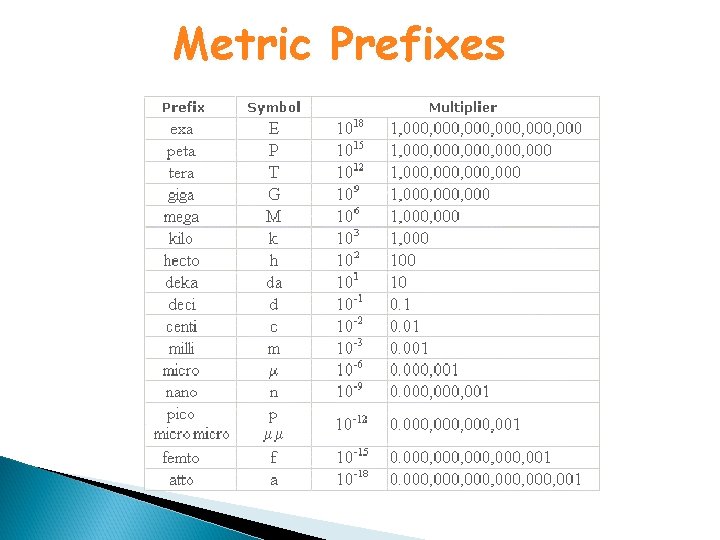
Metric Prefixes

Conversion factors Fractions in which the numerator and denominator are EQUAL quantities expressed in different units Dimensional Analysis (Factor-label method) A way of solving problems using conversion factors The UNITS ensure that you have the conversion factor in the proper arrangement

What is Scientific Notation? • A way of expressing really big or really small numbers • Most often used in “scientific” calculations where the analysis must be very precise

Scientific notation consists of two parts: • A number between 1 and 10 (N) • A power of 10 (number of decimal places moved) N x 10 x
Significant Figures § The numbers reported in a measurement are limited by the measuring devise § Shows the accuracy of the measuring tool used § Include all the known digits plus one estimated digit
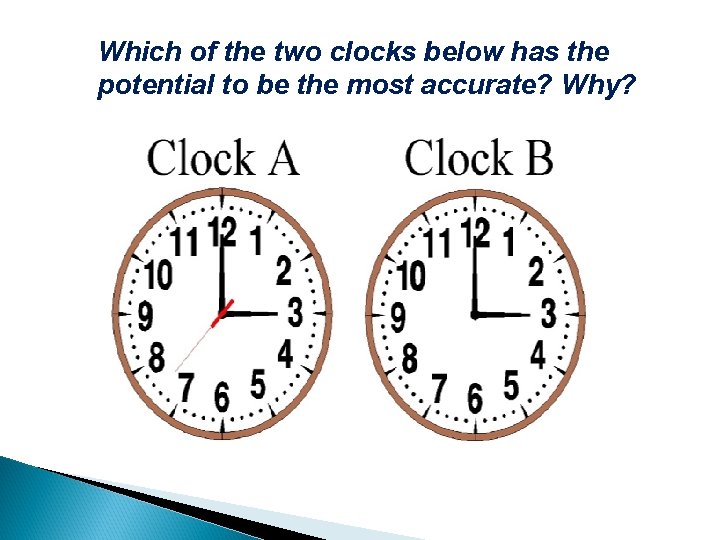
Which of the two clocks below has the potential to be the most accurate? Why?
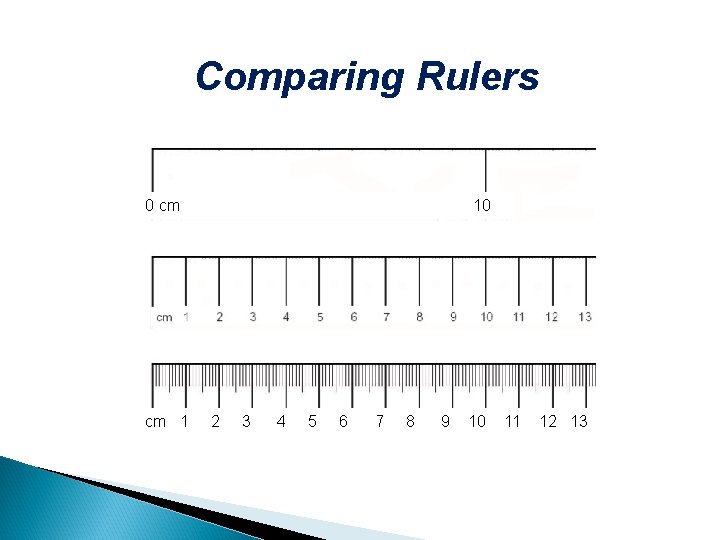
Comparing Rulers 0 cm cm 1 10 2 3 4 5 6 7 8 9 10 11 12 13
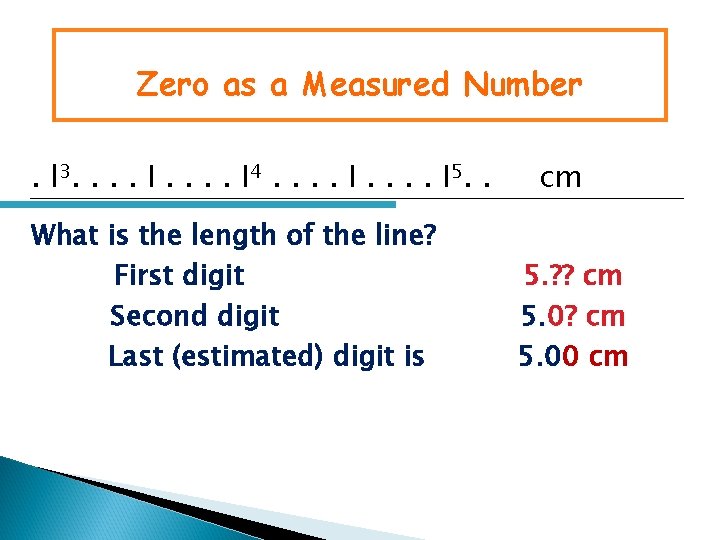
Zero as a Measured Number. l 3. . . . I 4. . . . I 5. . What is the length of the line? First digit Second digit Last (estimated) digit is cm 5. ? ? cm 5. 00 cm

Reading a Meterstick. l 2. . . . I 3. . . . I 4. . First digit (known) =2 cm 2. ? ? cm Second digit (known) = 0. 7 2. 7? cm Third digit (estimated) between 0. 05 - 0. 07 Length reported = 2. 75 cm or 2. 74 cm or 2. 76 cm
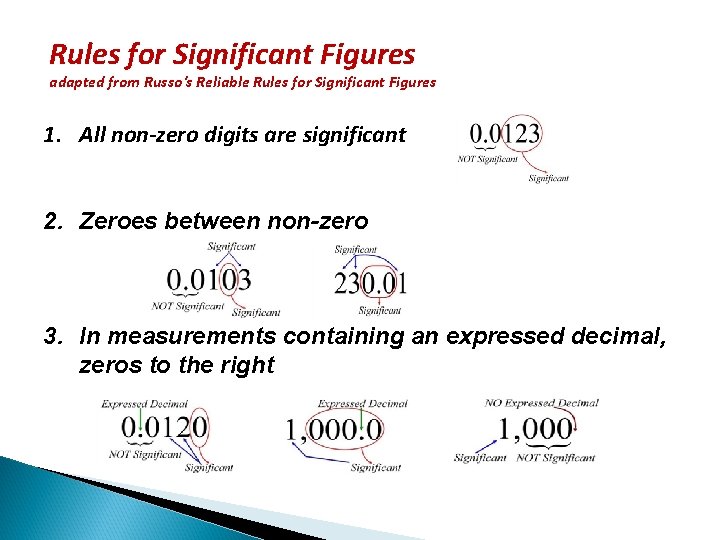
Rules for Significant Figures adapted from Russo's Reliable Rules for Significant Figures 1. All non-zero digits are significant 2. Zeroes between non-zero digits are significant 3. In measurements containing an expressed decimal, zeros to the right of NON-ZERO digits are significant.
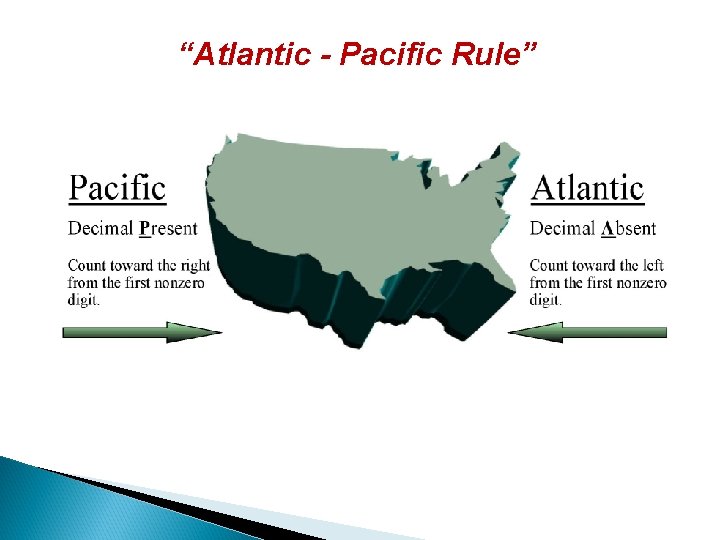
“Atlantic - Pacific Rule” Count from the ocean towards the coast starting with the first nonzero digit, and include all the digits that follow.

Significant Numbers in Calculations § A calculated answer cannot be more accurate than the measuring tool used § Must match the least accurate measurement § Significant figures are needed for all final answers and determine where to round Two rules for rounding: 1) adding or subtracting 2) multiplying or dividing
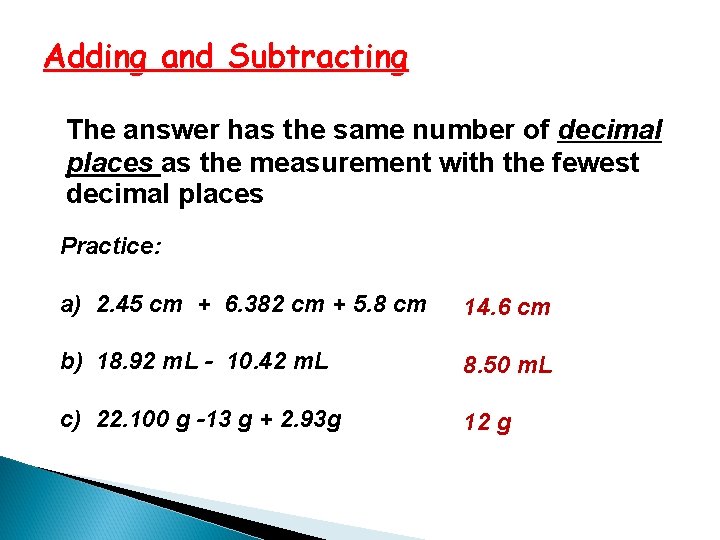
Adding and Subtracting The answer has the same number of decimal places as the measurement with the fewest decimal places Practice: a) 2. 45 cm + 6. 382 cm + 5. 8 cm 14. 6 cm b) 18. 92 m. L - 10. 42 m. L 8. 50 m. L c) 22. 100 g -13 g + 2. 93 g 12 g

Multiplying and Dividing The calculated answer should have the same number of significant figures as the measurement with the fewest sig. figs. Practice: a) 4. 20 m x 3. 21 m x 0. 16 m 2. 2 m 3 b) 100. 35 g / 90. 2 m. L 1. 11 g/m. L c) (3. 28 x 10 -5 km) / (4 x 102 s) 8 x 10 -8 km/s
Temperature Scales • Fahrenheit • Celsius • Kelvin Anders Celsius 1701 -1744 Lord Kelvin (William Thomson) 1824 -1907
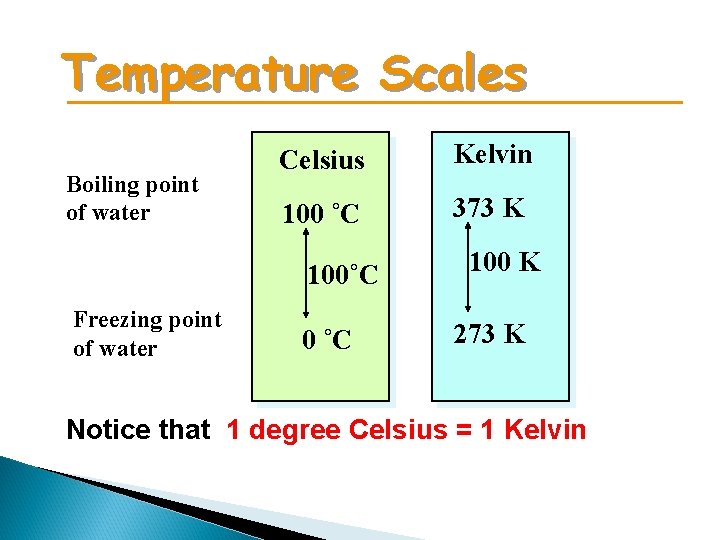
Temperature Scales Boiling point of water Celsius Kelvin 100 ˚C 373 K 100˚C Freezing point of water 0 ˚C 100 K 273 K Notice that 1 degree Celsius = 1 Kelvin
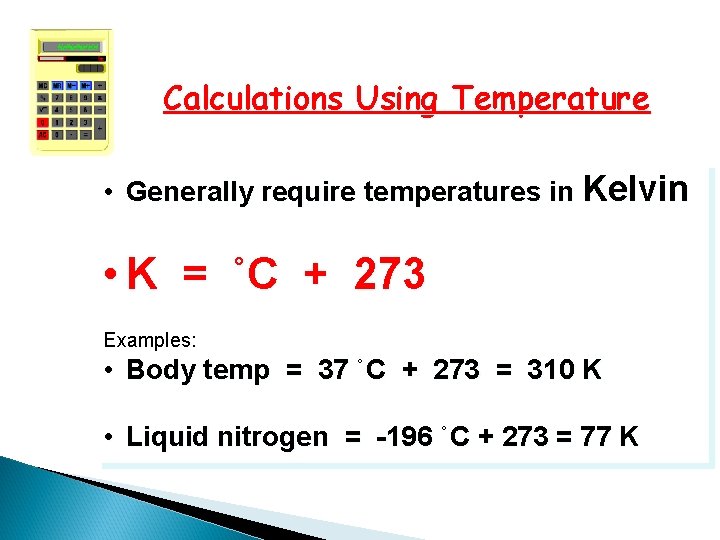
Calculations Using Temperature • Generally require temperatures in Kelvin • K = ˚C + 273 Examples: • Body temp = 37 ˚C + 273 = 310 K • Liquid nitrogen = -196 ˚C + 273 = 77 K
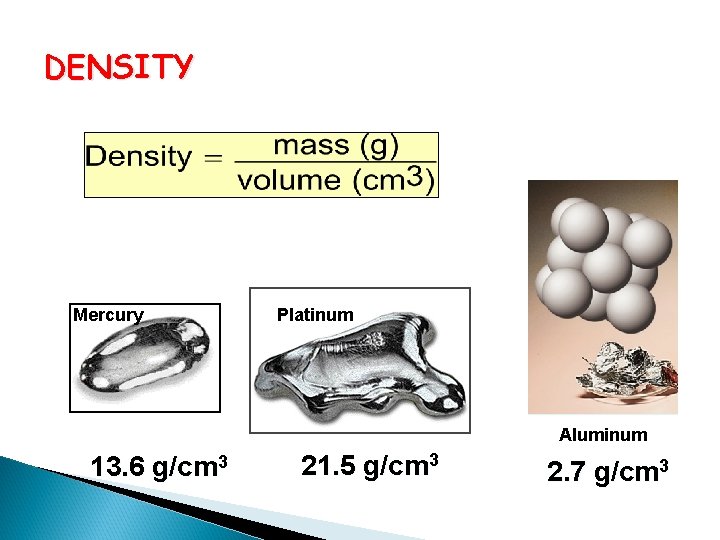
DENSITY Mercury Platinum Aluminum 13. 6 g/cm 3 21. 5 g/cm 3 2. 7 g/cm 3
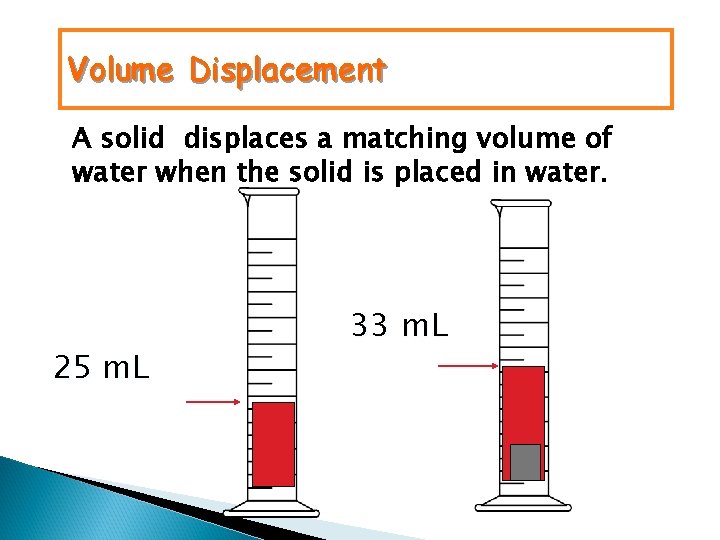
Volume Displacement A solid displaces a matching volume of water when the solid is placed in water. 25 m. L 33 m. L
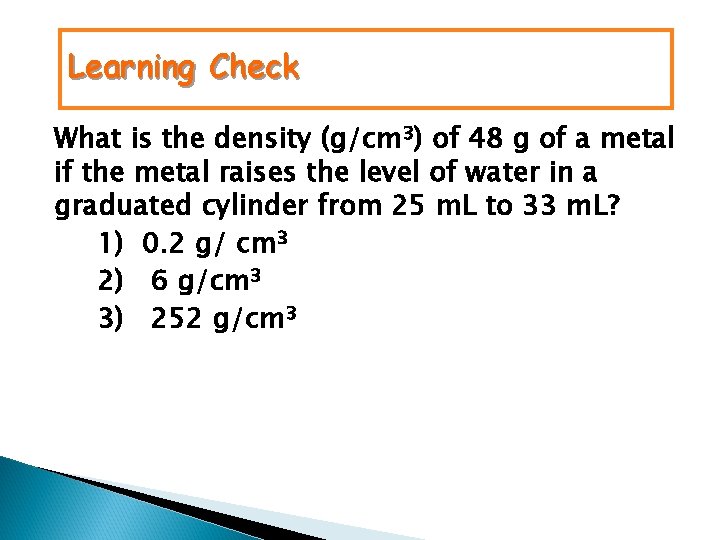
Learning Check What is the density (g/cm 3) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 m. L to 33 m. L? 1) 0. 2 g/ cm 3 2) 6 g/cm 3 3) 252 g/cm 3
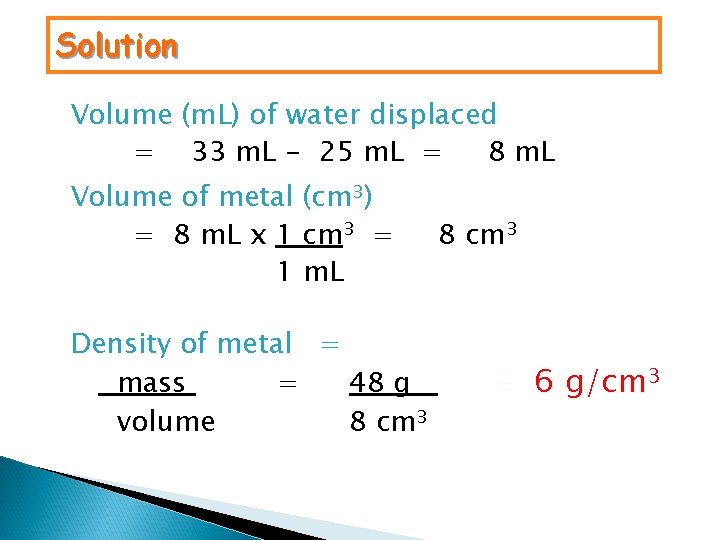
Solution Volume (m. L) of water displaced = 33 m. L - 25 m. L = 8 m. L Volume of metal (cm 3) = 8 m. L x 1 cm 3 = 1 m. L Density of metal = mass = 48 g volume 8 cm 3 = 6 g/cm 3
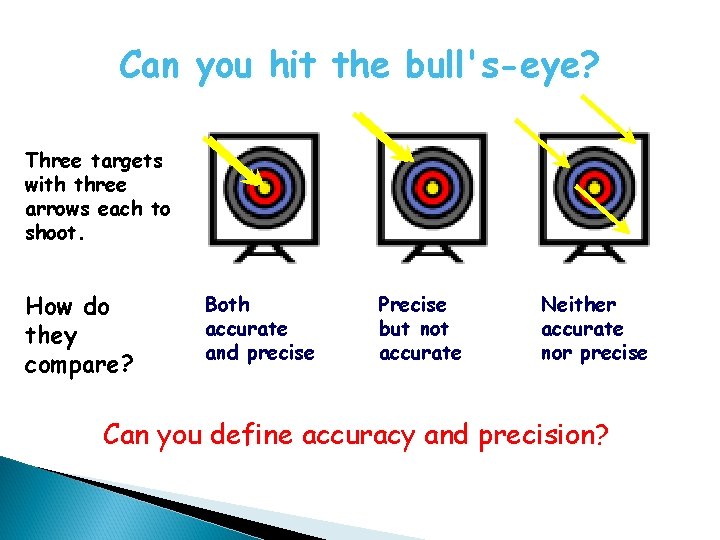
Can you hit the bull's-eye? Three targets with three arrows each to shoot. How do they compare? Both accurate and precise Precise but not accurate Neither accurate nor precise Can you define accuracy and precision?
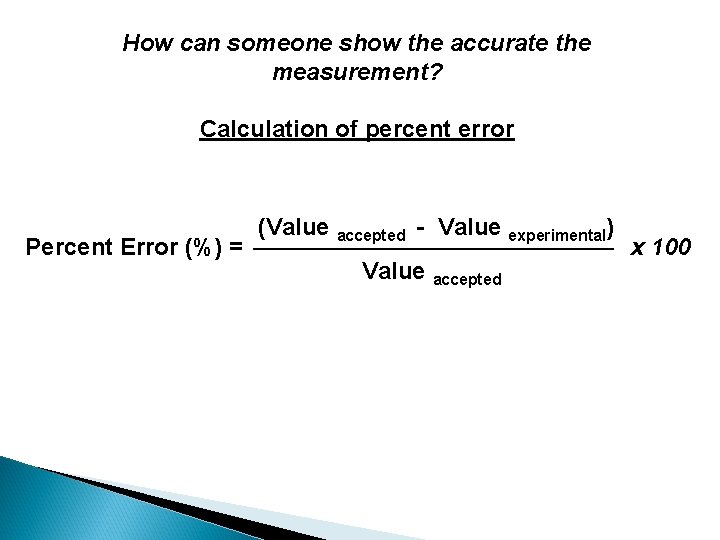
How can someone show the accurate the measurement? Calculation of percent error Percent Error (%) = (Value accepted - Value experimental) Value accepted x 100
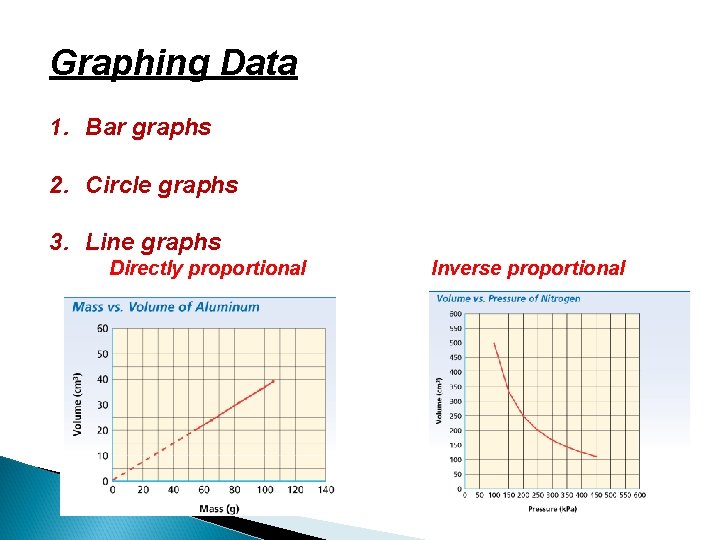
Graphing Data 1. Bar graphs 2. Circle graphs 3. Line graphs Directly proportional Inverse proportional
- Slides: 26