Measurements l l l Scientists use two word


- Slides: 23

Measurements l l l Scientists use two word to describe how good the measurements are Accuracy- how close the measurement is to the actual value Precision- how well can the measurement be repeated

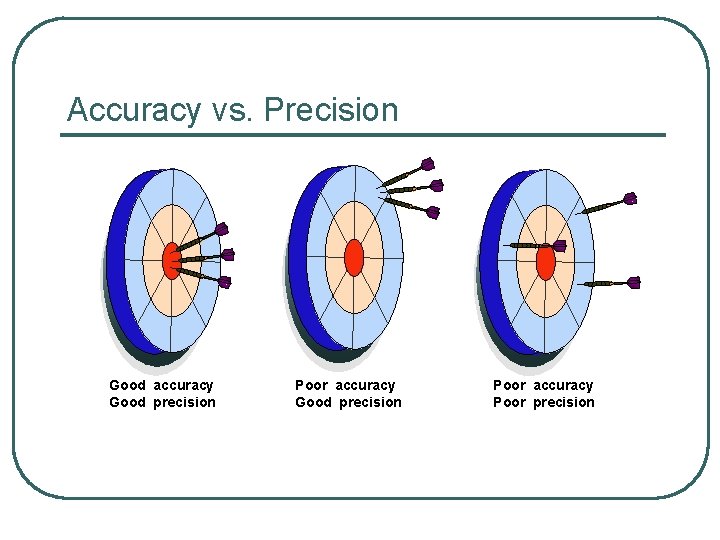
Accuracy vs. Precision Good accuracy Good precision Poor accuracy Poor precision

Differences l l Accuracy can be true of an individual measurement or the average of several Precision requires several measurements before anything can be said about it

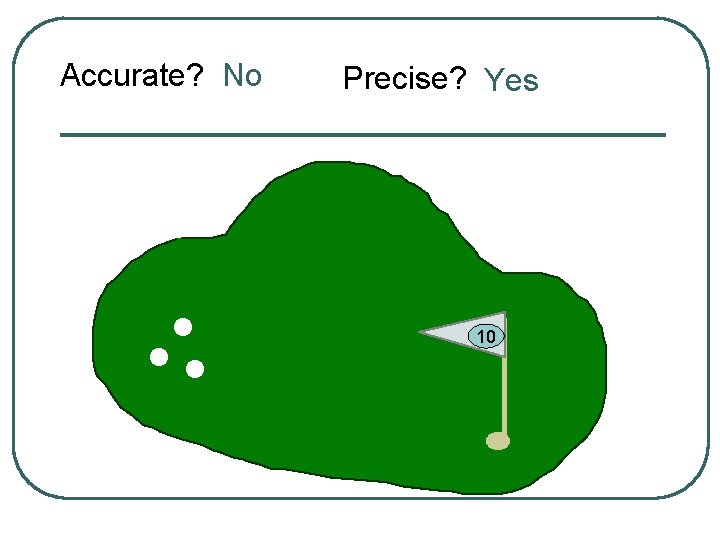
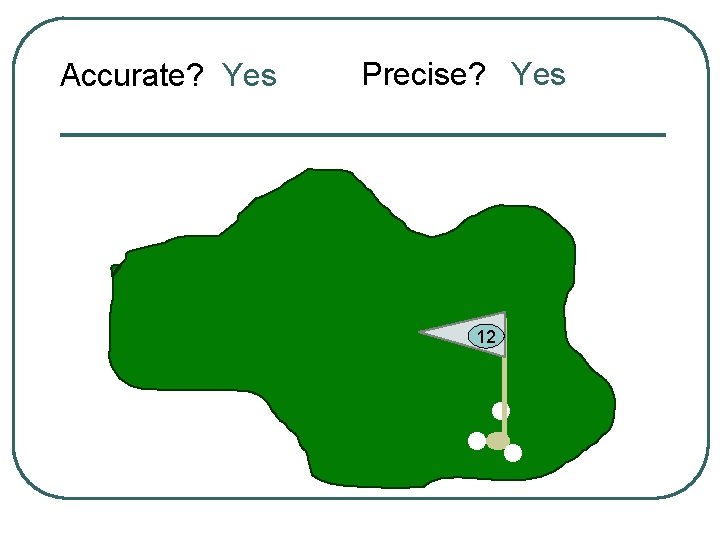
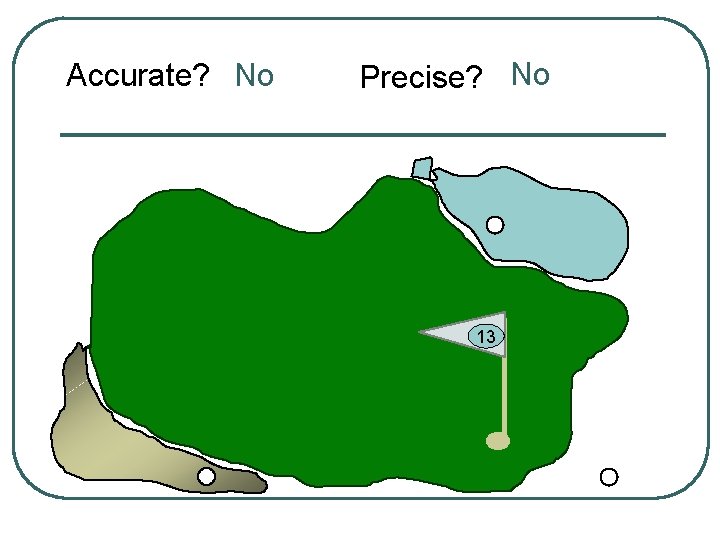
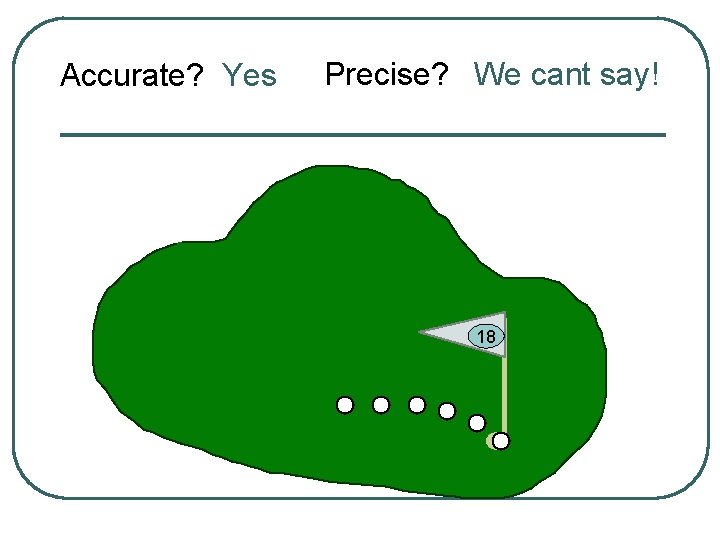
Let’s use a golf anaolgy

Accurate? No Precise? Yes 10

Accurate? Yes Precise? Yes 12

Accurate? No Precise? No 13

Accurate? Yes Precise? We cant say! 18

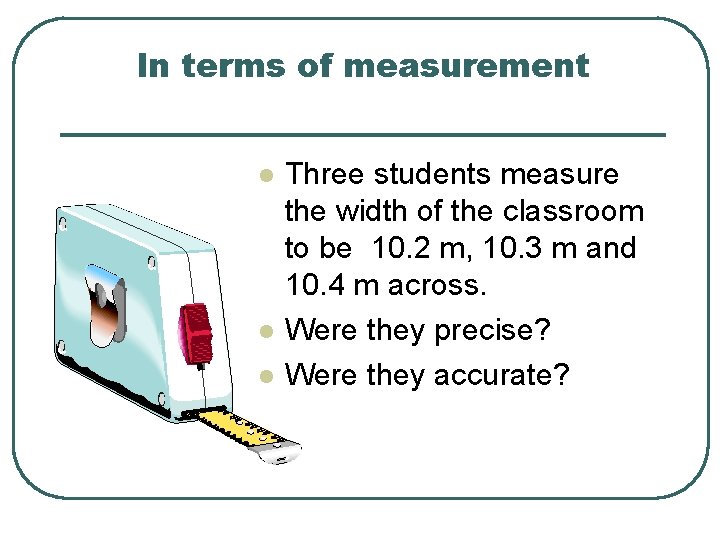
Accuracy vs. Precision l Accuracy how close a measurement is to the accepted value l Precision - how close a series of measurements are to each other ACCURATE = CORRECT PRECISE = CONSISTENT(Reproducible)

In terms of measurement l l l Three students measure the width of the classroom to be 10. 2 m, 10. 3 m and 10. 4 m across. Were they precise? Were they accurate?

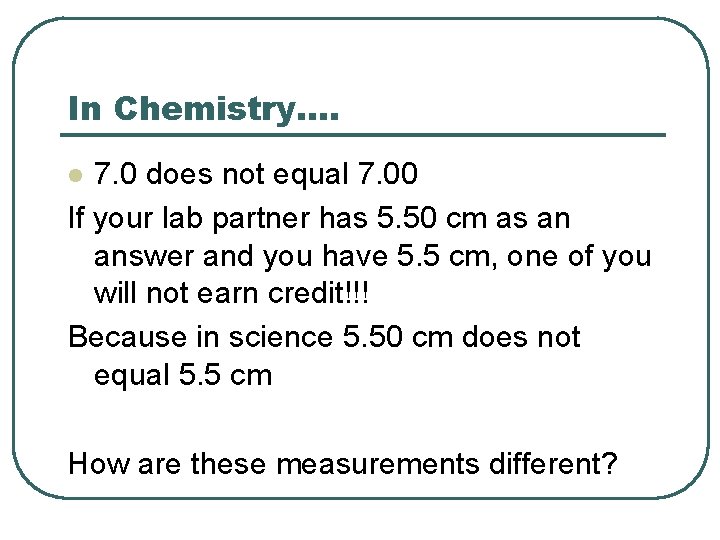
In Chemistry…. 7. 0 does not equal 7. 00 If your lab partner has 5. 50 cm as an answer and you have 5. 5 cm, one of you will not earn credit!!! Because in science 5. 50 cm does not equal 5. 5 cm l How are these measurements different?

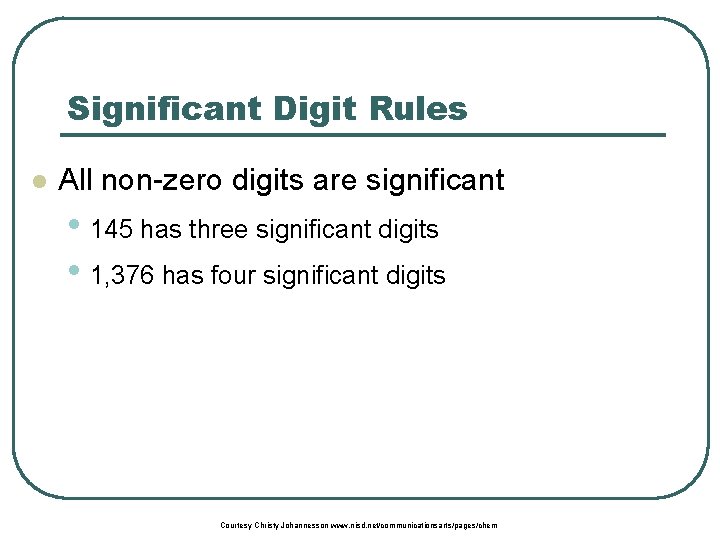
Significant Digit Rules l All non-zero digits are significant • 145 has three significant digits • 1, 376 has four significant digits Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

2. Zeros a. Leading Zeros – NEVER significant b. Middle Zeros – ALWAYS significant c. Trailing Zeros – SOMETIMES significant (Depends on the presence of a decimal point) i. If decimal point is present: IS significant ii. If decimal point is not present: IS NOT significant

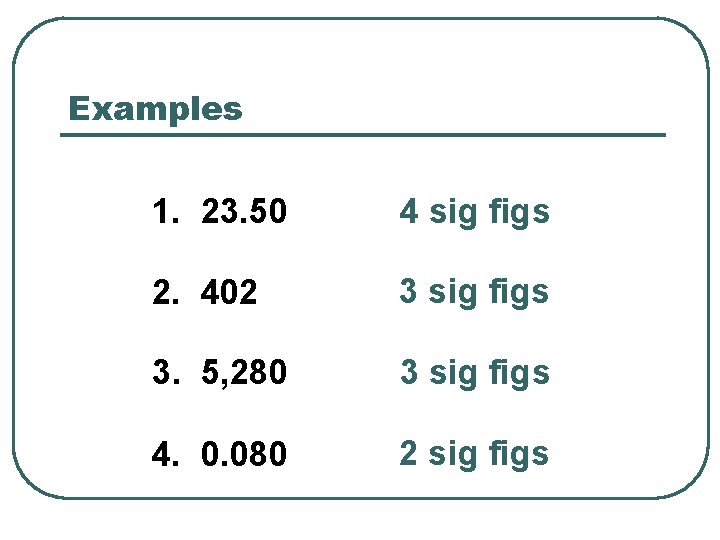
Examples 1. 23. 50 4 sig figs 2. 402 3 sig figs 3. 5, 280 3 sig figs 4. 0. 080 2 sig figs

Let’s Practice!!! l Take 2 minutes to answer the practice problems at the bottom of the page. l Put your pencil down when you are finished and we can check our answers together.

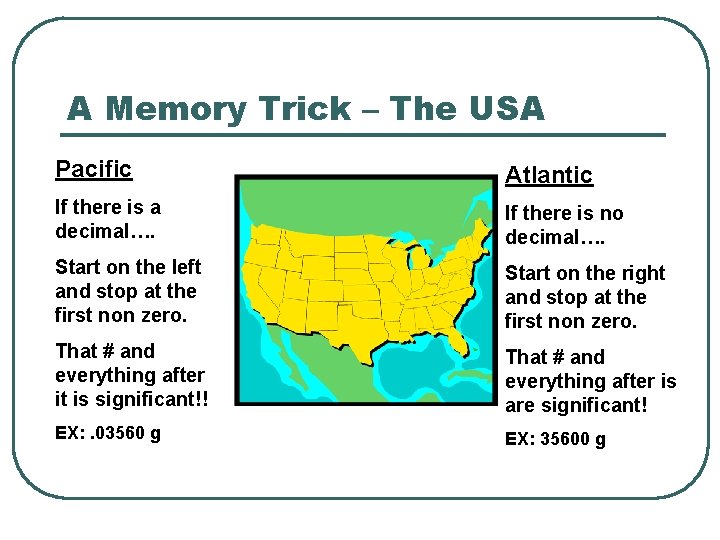
A Memory Trick – The USA Pacific Atlantic If there is a decimal…. If there is no decimal…. Start on the left and stop at the first non zero. Start on the right and stop at the first non zero. That # and everything after it is significant!! That # and everything after is are significant! EX: . 03560 g EX: 35600 g

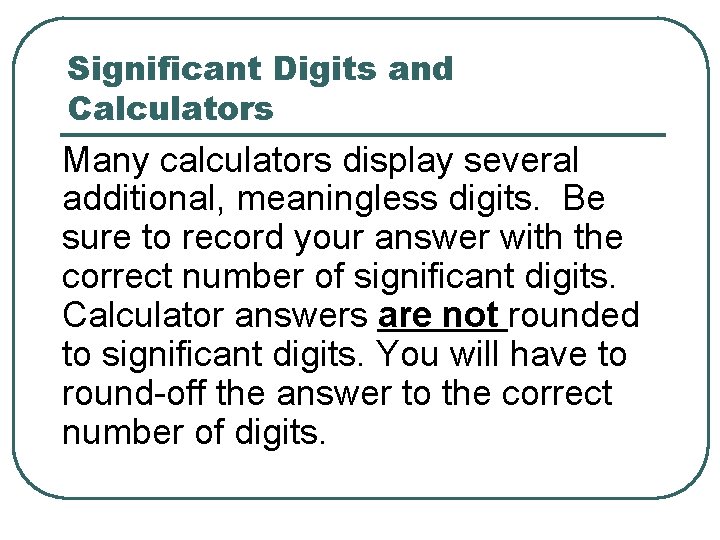
Significant Digits and Calculators Many calculators display several additional, meaningless digits. Be sure to record your answer with the correct number of significant digits. Calculator answers are not rounded to significant digits. You will have to round-off the answer to the correct number of digits.

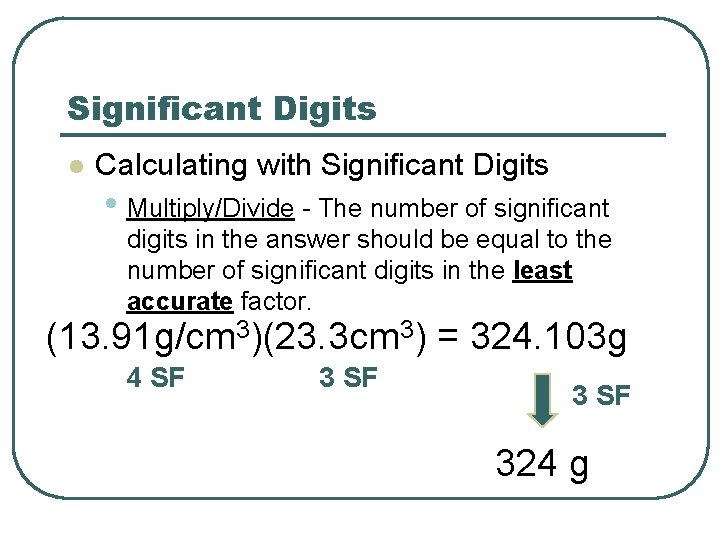
Significant Digits l Calculating with Significant Digits • Multiply/Divide - The number of significant digits in the answer should be equal to the number of significant digits in the least accurate factor. (13. 91 g/cm 3)(23. 3 cm 3) = 324. 103 g 4 SF 324 g

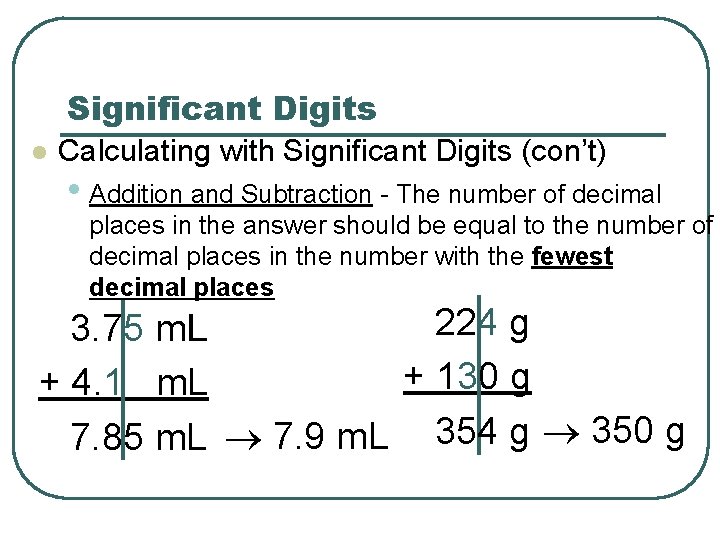
Significant Digits l Calculating with Significant Digits (con’t) • Addition and Subtraction - The number of decimal places in the answer should be equal to the number of decimal places in the number with the fewest decimal places 224 g 3. 75 m. L + 130 g + 4. 1 m. L 7. 85 m. L 7. 9 m. L 354 g 350 g

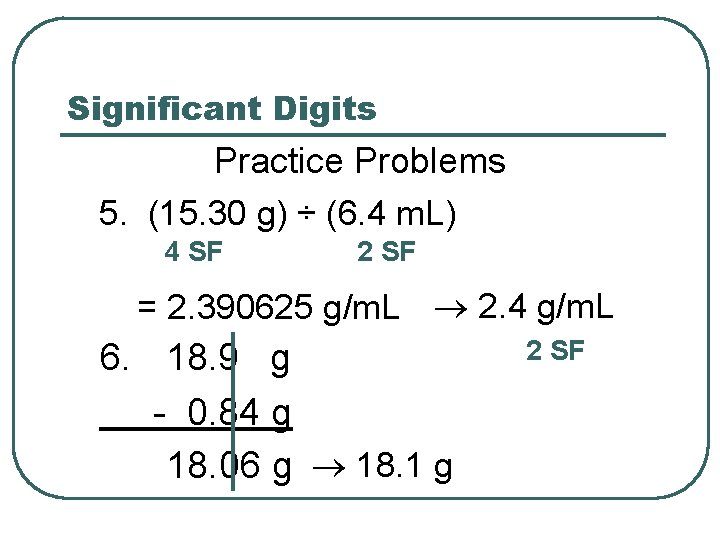
Significant Digits Practice Problems 5. (15. 30 g) ÷ (6. 4 m. L) 4 SF 2 SF = 2. 390625 g/m. L 2. 4 g/m. L 6. 18. 9 g - 0. 84 g 18. 1 g 18. 06 g 2 SF

Reporting Measurements l Using significant figures l Report what is known with certainty l Add ONE digit of uncertainty (estimation)

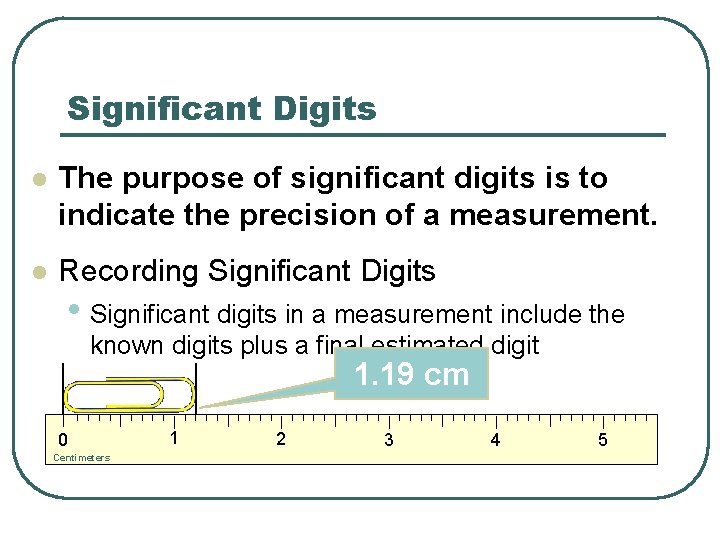
Significant Digits l The purpose of significant digits is to indicate the precision of a measurement. l Recording Significant Digits • Significant digits in a measurement include the known digits plus a final estimated digit 1. 19 cm 0 Centimeters 1 2 3 4 5

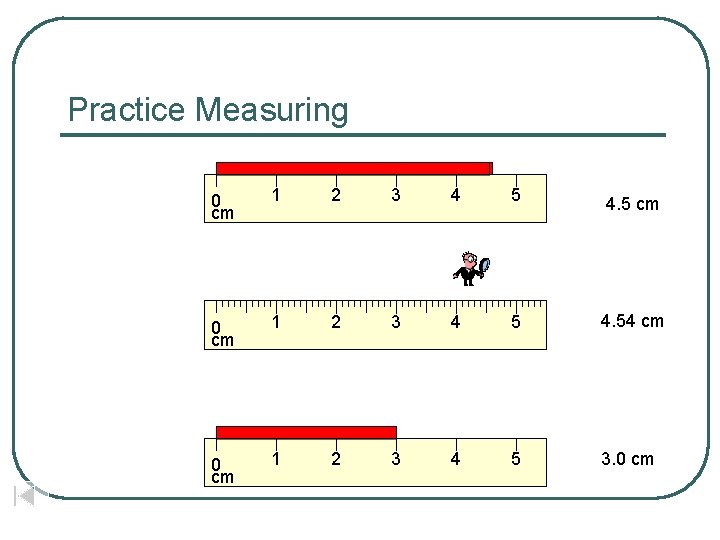
Practice Measuring 0 cm 1 2 3 4 5 4. 5 cm 0 cm 1 2 3 4 5 4. 54 cm 0 cm 1 2 3 4 5 3. 0 cm