MEASUREMENTS HTTP WWW BRAINPOP COMSCIENCEMATTERANDCHEMISTRYMEASURINGMA TTERPREVIEW WEML MEASUREMENT

MEASUREMENTS HTTP: //WWW. BRAINPOP. COM/SCIENCE/MATTERANDCHEMISTRY/MEASURINGMA TTER/PREVIEW. WEML
MEASUREMENT IN CHEMISTRY WE ¨DO EXPERIMENTS ¨MEASURE QUANTITIES ¨USE NUMBERS TO REPORT MEASUREMENTS

METRIC SYSTEM (SI) • • • IS A DECIMAL SYSTEM BASED ON 10 USED IN MOST OF THE WORLD USED BY SCIENTISTS AND HOSPITALS * SI means System International
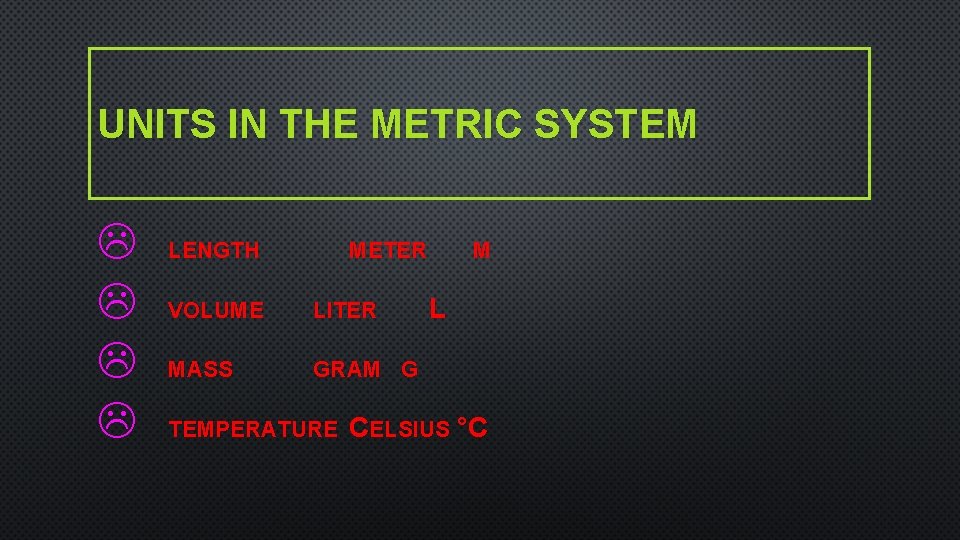
UNITS IN THE METRIC SYSTEM L L LENGTH METER VOLUME LITER MASS GRAM G TEMPERATURE M L CELSIUS °C
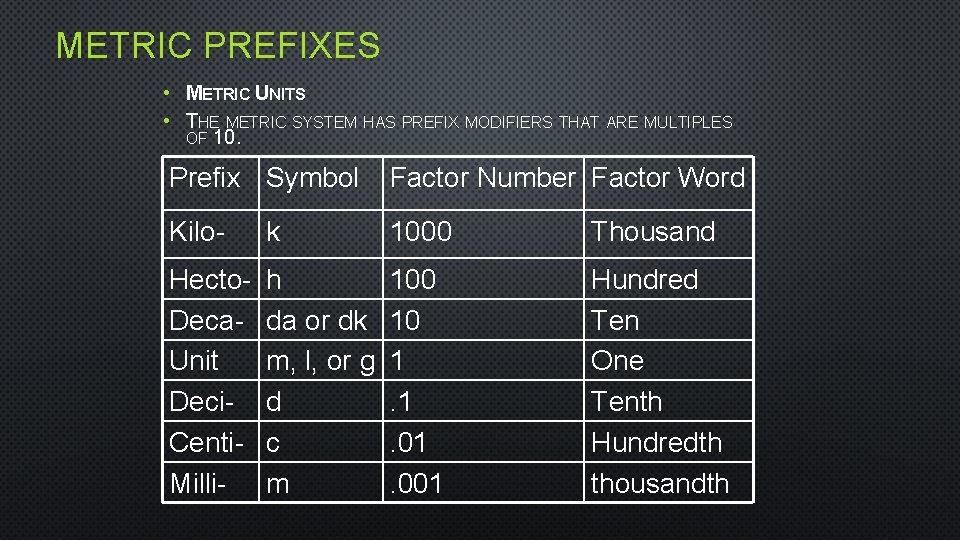
METRIC PREFIXES • METRIC UNITS • THE METRIC SYSTEM HAS PREFIX MODIFIERS THAT ARE MULTIPLES OF 10. Prefix Symbol Factor Number Factor Word Kilo- k 1000 Thousand Hecto. Deca. Unit Deci. Centi. Milli- h da or dk m, l, or g d c m 100 10 1. 1. 001 Hundred Ten One Tenth Hundredth thousandth
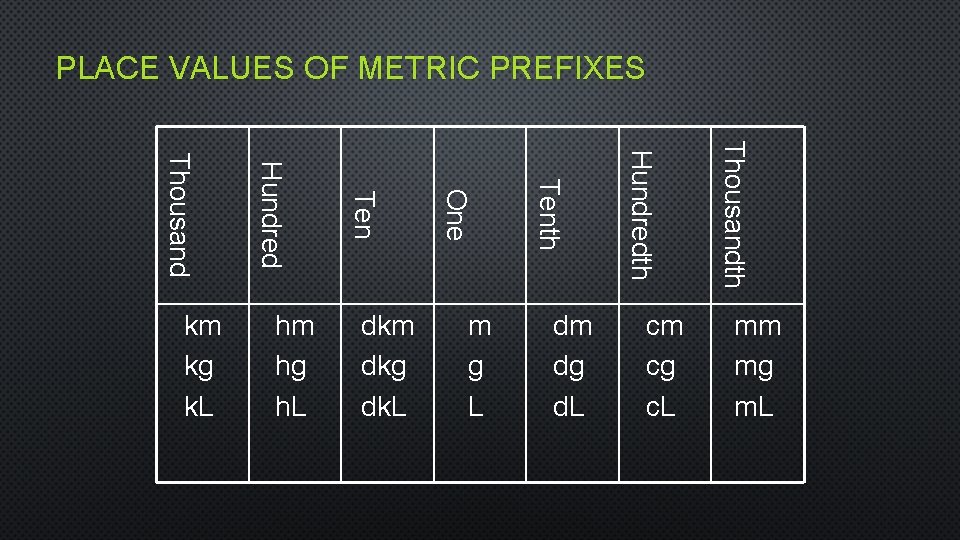
PLACE VALUES OF METRIC PREFIXES cm cg c. L Thousandth dm dg d. L Hundredth m g L Tenth dkm dkg dk. L One hm hg h. L Ten Hundred Thousand km kg k. L mm mg m. L
STATING A MEASUREMENT IN EVERY MEASUREMENT THERE IS A ¨NUMBER FOLLOWED BY A ¨ UNIT FROM A MEASURING DEVICE THE NUMBER SHOULD ALSO BE AS PRECISE AS THE MEASUREMENT!

UNCERTAINTY IN MEASUREMENT • SCIENTIFIC MEASUREMENTS ARE REPORTED SO THAT EVERY DIGIT IS CERTAIN EXCEPT THE LAST, WHICH IS ESTIMATED.
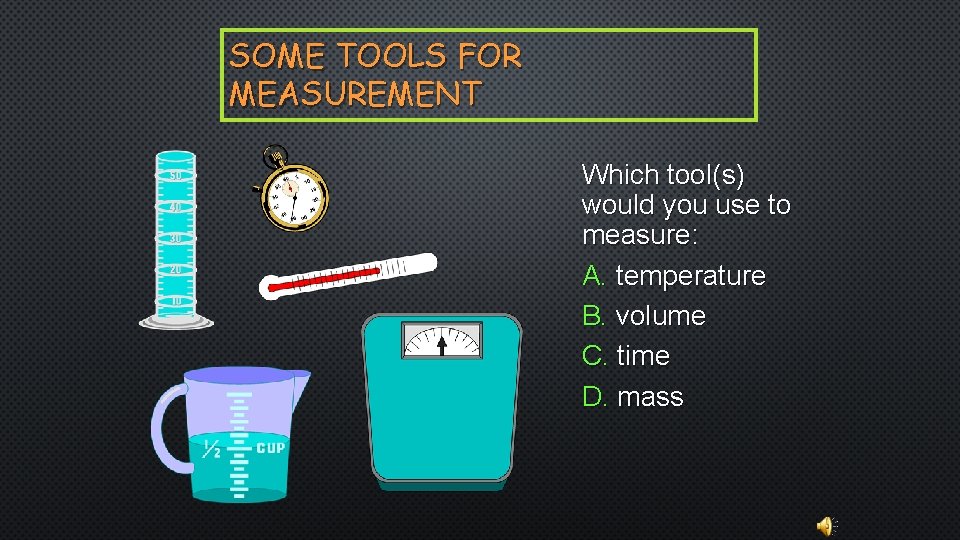
SOME TOOLS FOR MEASUREMENT Which tool(s) would you use to measure: A. temperature B. volume C. time D. mass

When we measure length we measure how long something is. You can measure the length of ANYTHING!
Standard units Millimeters (mm) Meters (m) Centimeters (cm) Kilometers (km)

MASS VS. WEIGHT • MASS: AMOUNT OF MATTER (GRAMS, MEASURED WITH A BALANCE) • WEIGHT: FORCE EXERTED BY THE MASS, ONLY PRESENT WITH GRAVITY (POUNDS, MEASURED WITH A SCALE) Can you hear me now?
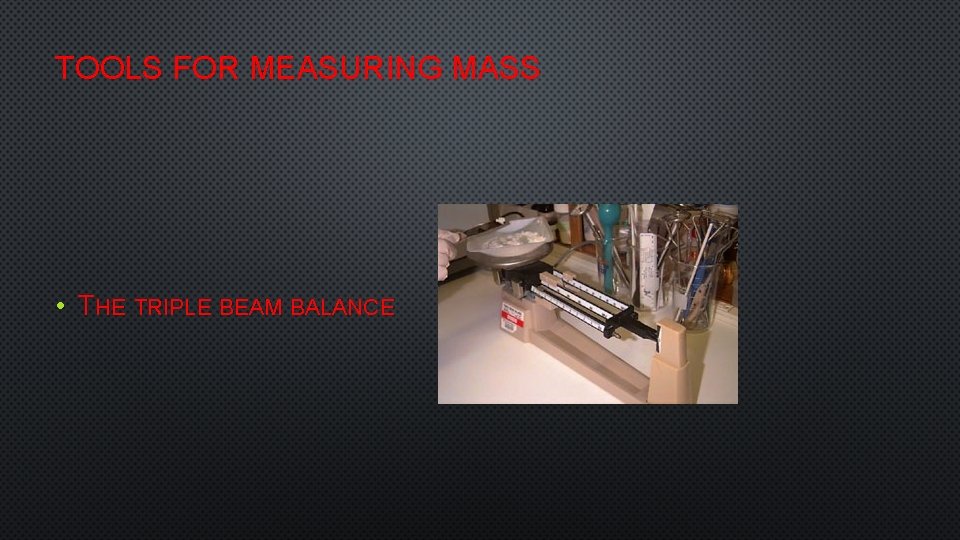
TOOLS FOR MEASURING MASS • THE TRIPLE BEAM BALANCE

HOW TO USE A TRIPLE BEAM BALANCE • 1) MAKE SURE THAT THE BALANCE SCALE IS AT ZERO. • 2) PLACE OBJECT ON BALANCE SCALE THE LARGES AND MOVE THE LARGEST RIDER ONE NOTCH AT A TIME UNTIL THE BEAM DROPS. MOVE THE RIDER BACK ONE NOTCH (MAKE SURE IT LOCK IN PLACE) • 3) MOVE THE NEXT LARGEST RIDER UNTIL THE BEAM DROPS. BACK IT UP ONE NOTCH. • 4) MOVE THE SMALLEST RIDER UNTIL THE BEAM SWINGS EQUALLY ABOVE AND BELOW THE ZERO MARK. • 5) ADD UP ALL THE NUMBERS TO FIND THE MASS!

WHAT IS VOLUME? • VOLUME IS THE MEASURE OF THE SPACE SOMETHING TAKES UP. • IT IS THE MEASURE OF HOW MUCH A CONTAINER OF A PARTICULAR SHAPE WILL HOLD - LIQUIDS, DRY SUBSTANCES, ETC.

WHAT’S THE FORMULA? The formula for finding the volume of a rectangle is …. L x W x H = Volume This means we take the length times the width, then multiply that by the height.
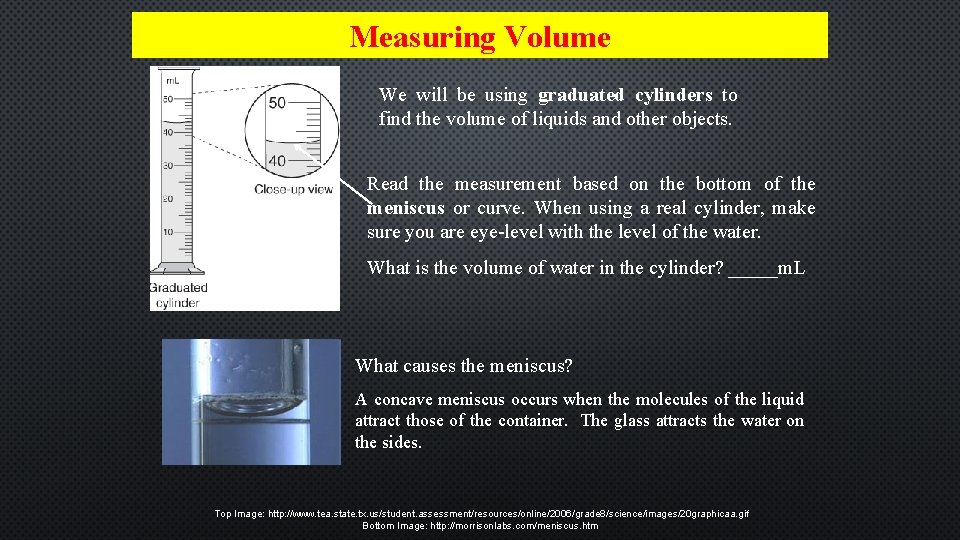
Measuring Volume We will be using graduated cylinders to find the volume of liquids and other objects. Read the measurement based on the bottom of the meniscus or curve. When using a real cylinder, make sure you are eye-level with the level of the water. What is the volume of water in the cylinder? _____m. L What causes the meniscus? A concave meniscus occurs when the molecules of the liquid attract those of the container. The glass attracts the water on the sides. Top Image: http: //www. tea. state. tx. us/student. assessment/resources/online/2006/grade 8/science/images/20 graphicaa. gif Bottom Image: http: //morrisonlabs. com/meniscus. htm
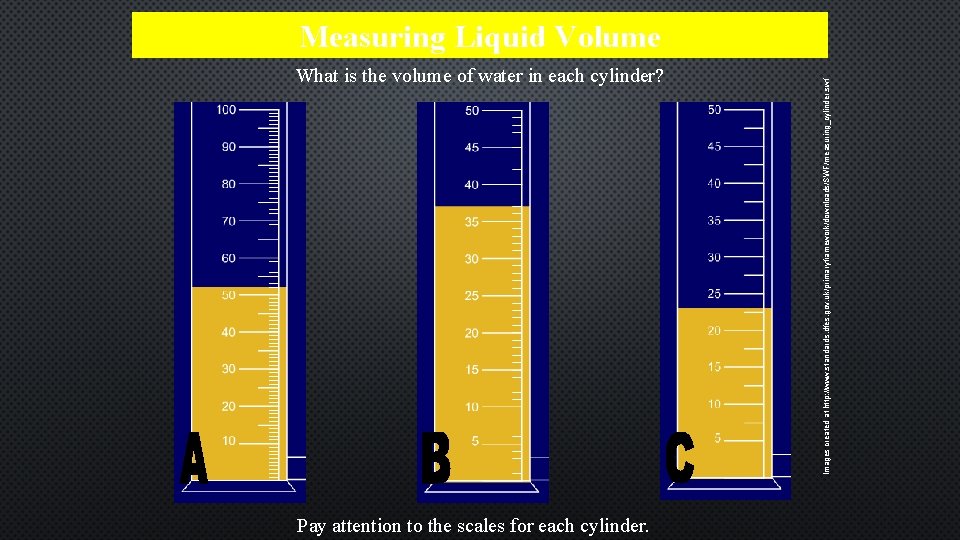
What is the volume of water in each cylinder? Pay attention to the scales for each cylinder. Images created at http: //www. standards. dfes. gov. uk/primaryframework/downloads/SWF/measuring_cylinder. swf Measuring Liquid Volume
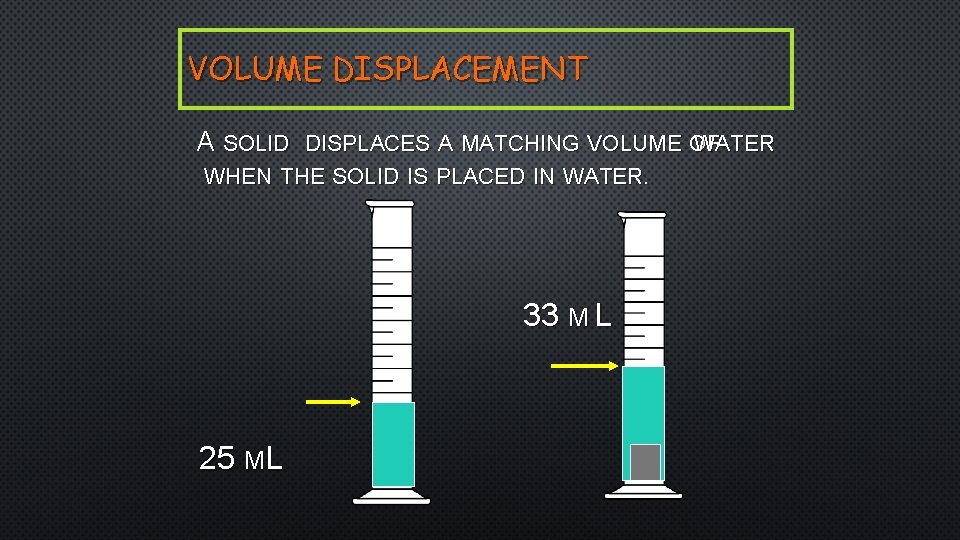
VOLUME DISPLACEMENT A SOLID DISPLACES A MATCHING VOLUME OF WATER WHEN THE SOLID IS PLACED IN WATER. 33 M L 25 ML
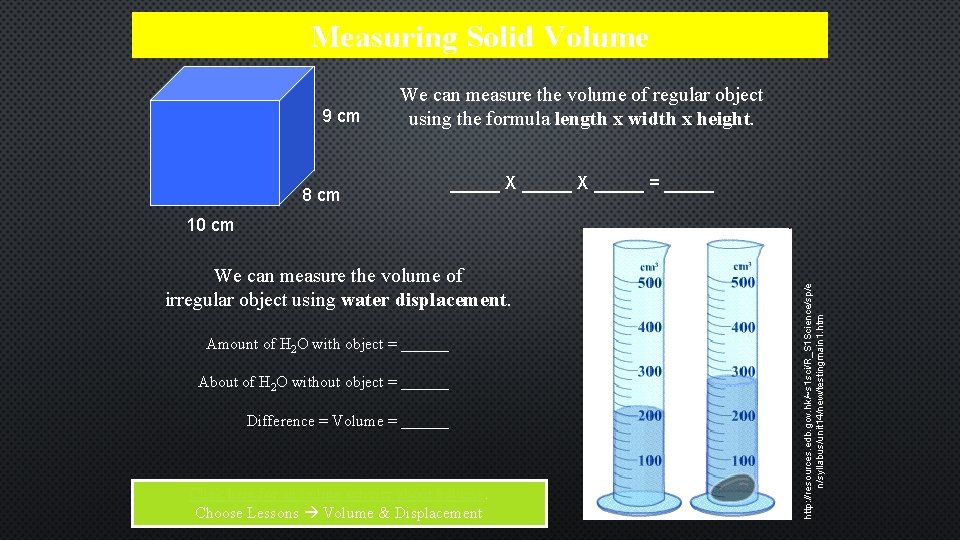
Measuring Solid Volume 9 cm We can measure the volume of regular object using the formula length x width x height. 8 cm _____ X _____ = _____ We can measure the volume of irregular object using water displacement. Amount of H 2 O with object = ______ About of H 2 O without object = ______ Difference = Volume = ______ Click here for an online activity about volume. Choose Lessons Volume & Displacement http: //resources. edb. gov. hk/~s 1 sci/R_S 1 Science/sp/e n/syllabus/unit 14/new/testingmain 1. htm 10 cm
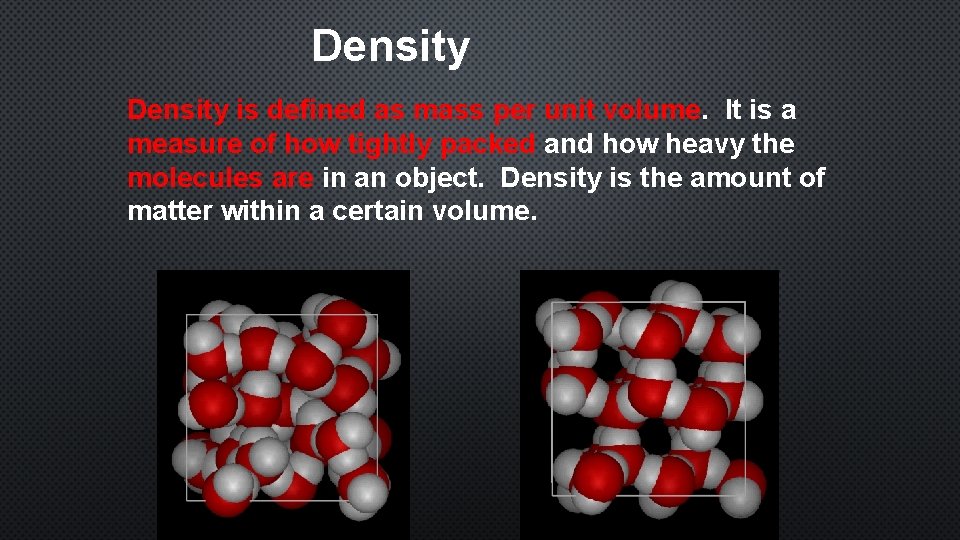
Density is defined as mass per unit volume. It is a measure of how tightly packed and how heavy the molecules are in an object. Density is the amount of matter within a certain volume.
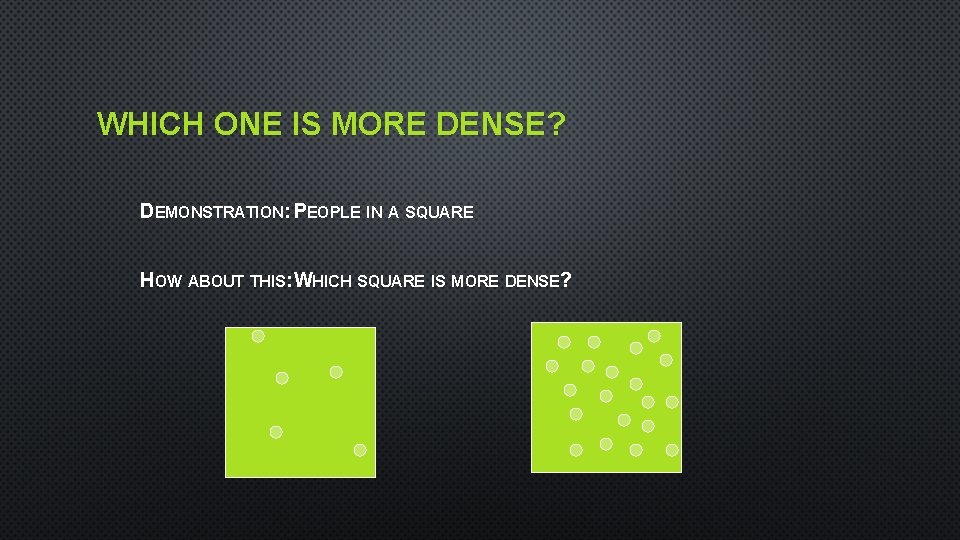
WHICH ONE IS MORE DENSE? DEMONSTRATION: PEOPLE IN A SQUARE HOW ABOUT THIS: WHICH SQUARE IS MORE DENSE?
WHICH ONE IS MORE DENSE? HTTPS: //WWW. YOUTUBE. COM/WATCH? V= ZHL 68 D 9 BPIW

WATER AND ICE HAVE DIFFERENT DENSITIES WHICH ONE IS MORE DENSE?
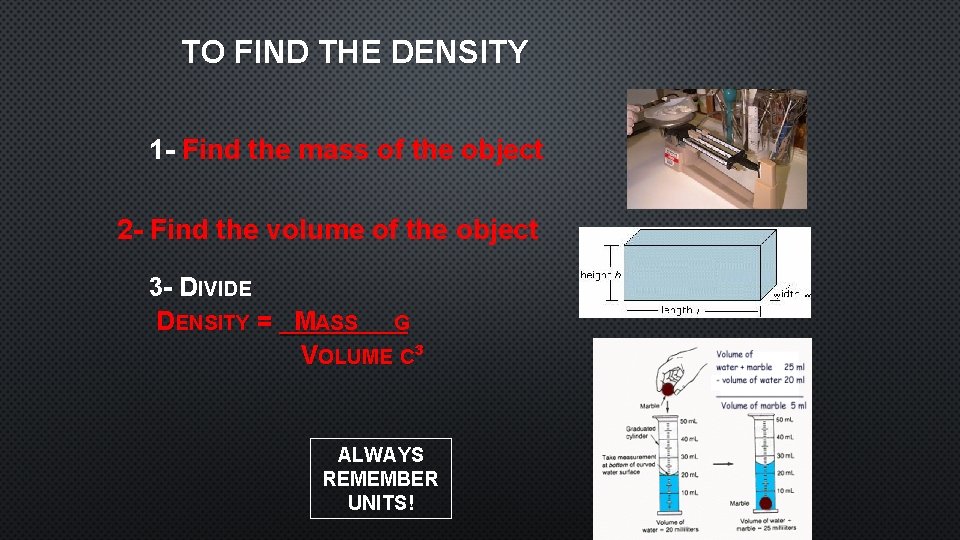
TO FIND THE DENSITY 1 - Find the mass of the object 2 - Find the volume of the object 3 - DIVIDE DENSITY = MASS G VOLUME C³ ALWAYS REMEMBER UNITS!
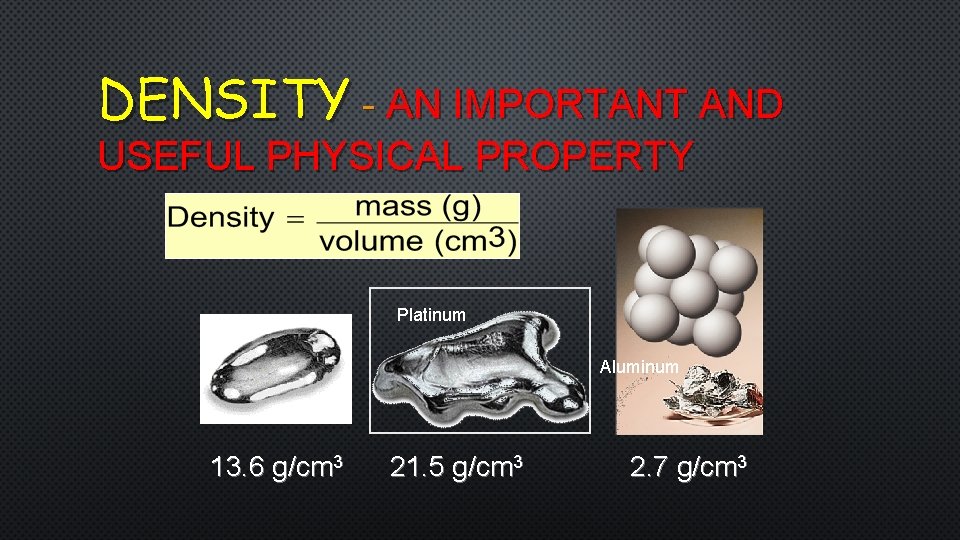
DENSITY - AN IMPORTANT AND USEFUL PHYSICAL PROPERTY Mercury Platinum Aluminum 13. 6 g/cm 3 21. 5 g/cm 3 2. 7 g/cm 3
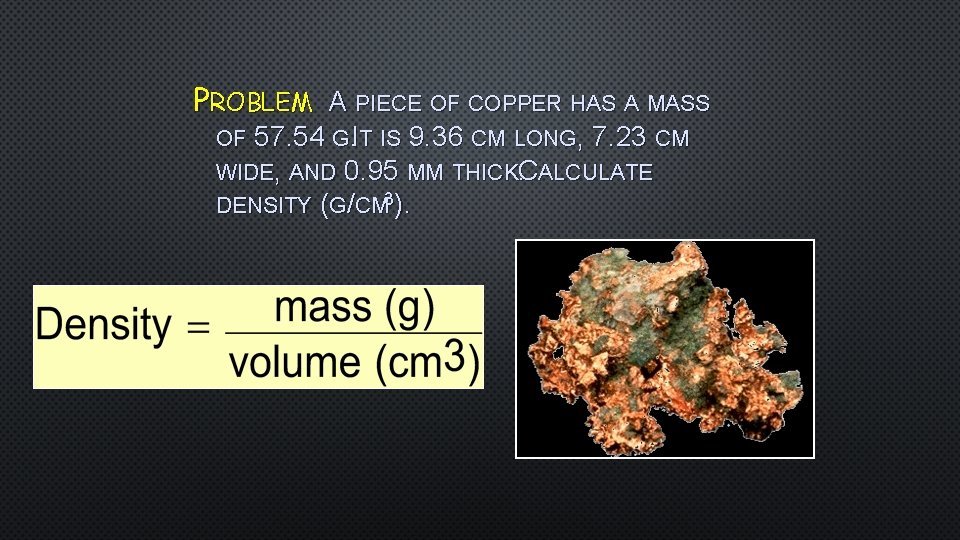
PROBLEM A PIECE OF COPPER HAS A MASS OF 57. 54 G. IT IS 9. 36 CM LONG, 7. 23 CM WIDE, AND 0. 95 MM THICKC. ALCULATE DENSITY (G/CM 3).

If you have 2 or more substances, the MORE dense substance will be on bottom The LESS dense substance will be on top

TEMPERATURE LPARTICLES ARE ALWAYS MOVING. LWHEN YOU HEAT WATER, THE WATER MOLECULES MOVE FASTER. LWHEN MOLECULES MOVE FASTER, THE SUBSTANCE GETS HOTTER. LWHEN A SUBSTANCE GETS HOTTER, ITS TEMPERATURE GOES UP. Lecture. PLUS Timberlake 31

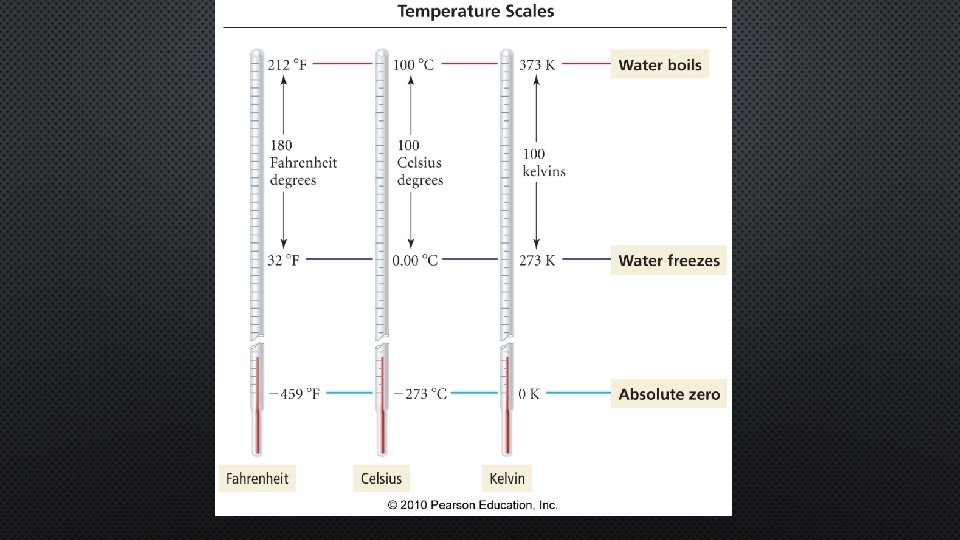
TEMPERATURE IS MEASURED IN DEGREES.
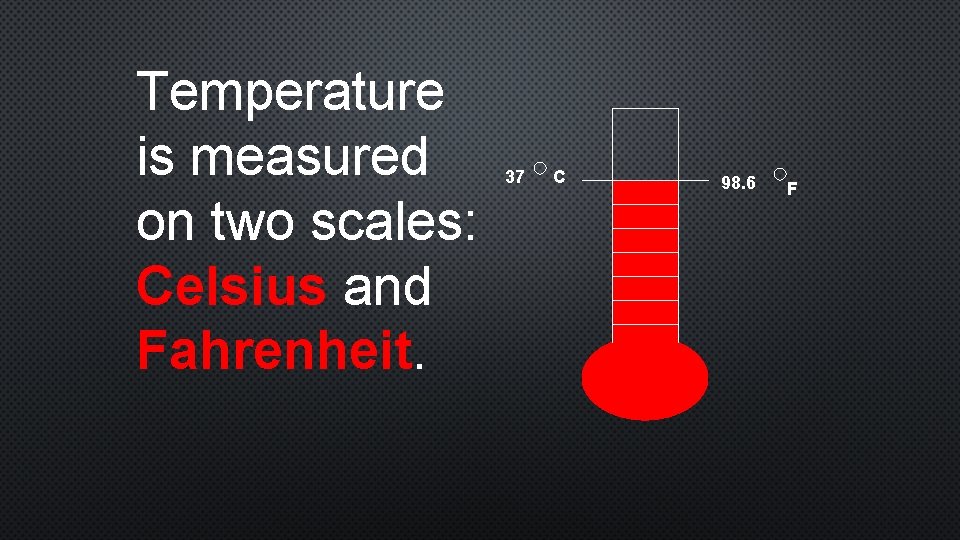
Temperature is measured on two scales: Celsius and Fahrenheit. 37 C 98. 6 F

We will use Celsius C The lower case “C” is the symbol for the Celsius scale.

- Slides: 34