Measurements Calculations Accuracy Precision Error Scientific Notation Accuracy






- Slides: 7

Measurements & Calculations Accuracy, Precision, % Error, Scientific Notation

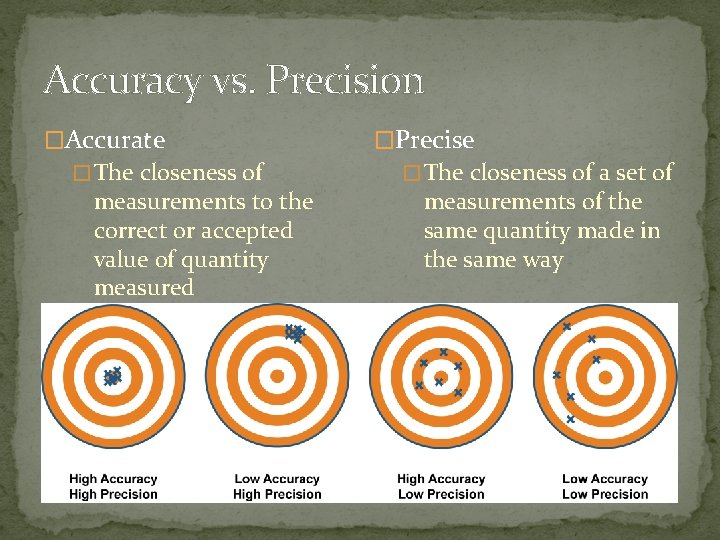
Accuracy vs. Precision �Accurate � The closeness of measurements to the correct or accepted value of quantity measured �Precise � The closeness of a set of measurements of the same quantity made in the same way

% Error �There a couple ways error could occur in your measurements: � Human Error – mistakes in experiment, measurements, etc. � Equipment Error – faulty or broken equipment, etc. �Calculating Error � Percentage error is calculated by subtracting the accepted value from the experimental value, dividing the difference by the accepted value, and then multiplying by 100. �Accepted Value – true/correct value (look it up!) �Experiment Value – measured in the experiment �Error = Accepted Value – Experimental Value

Scientific Notation � Numbers are written in the form M x 10 n, where the factor M is a number greater than or equal to 1 but less than 10, and n is a whole number. � Coefficient – the number greater than or equal to 1 but less than 10 � Exponent – is the whole number that indicates how many times the coefficient must be multiplied by 10(+) or by 1/10(-) � 65, 000 km or 6. 5 x 104 km � 0. 00012 mm or 1. 2 x 10 -4 mm � Steps: � Determining M by moving the decimal point in the original number to the left or the right so that only one nonzero digit remains to the left of the decimal point. � Determine n by counting the number of places that you moved the decimal point. If you moved it to the left, n is positive. If you moved it to the right, n is negative.

Adding or Subtracting Scientific Notation �These operations can only be performed when the values have the same exponents, or n factor. If they do not, adjustments must be made to the values so that the exponents are equal. � M factors can be added or subtracted � The exponents of the answer remain the same �Or it may be adjusted to the M factor stays between 1 and 10 4. 2 x 104 kg + 7. 9 x 103 kg

Multiplying & Dividing Scientific Notation � Multiply coefficients and ADD exponents. (5. 23 x 106 mm)(7. 1 x 10 -2 mm) (5. 23 x 7. 1) x 104 = 37. 133 x 104 mm or 3. 71 x 105 mm 2 � The coefficients are divided, and the exponent of the denominator is subtracted from that of the numerator. 5. 44 x 107 g = 5. 44 x 107 -4 g/mol = 0. 6716 x 103 g/mol 8. 1 x 104 mol 8. 1 or 6. 7 x 102 g/mol

Homework �Measurement Review Worksheet �Study for your Chapter 2 Test next class!!!