Measurements and Their Uncertainty 3 1 Slide 1





























- Slides: 49

Measurements and Their Uncertainty 3. 1 Slide 1 of 48

3. 1 Measurements and Their Uncertainty On January 4, 2004, the Mars Exploration Rover Spirit landed on Mars. Each day of its mission, Spirit recorded measurements for analysis. In the chemistry laboratory, you must strive for accuracy and precision in your measurements. Slide 2 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Using and Expressing Measurements How do measurements relate to science? Slide 3 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Using and Expressing Measurements A measurement is a quantity that has both a number and a unit. Measurements are fundamental to the experimental sciences. For that reason, it is important to be able to make measurements and to decide whether a measurement is correct. Slide 4 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Using and Expressing Measurements In scientific notation, a given number is written as the product of two numbers: a coefficient and 10 raised to a power. The number of stars in a galaxy is an example of an estimate that should be expressed in scientific notation. Slide 5 of 48 © Copyright Pearson Prentice Hall

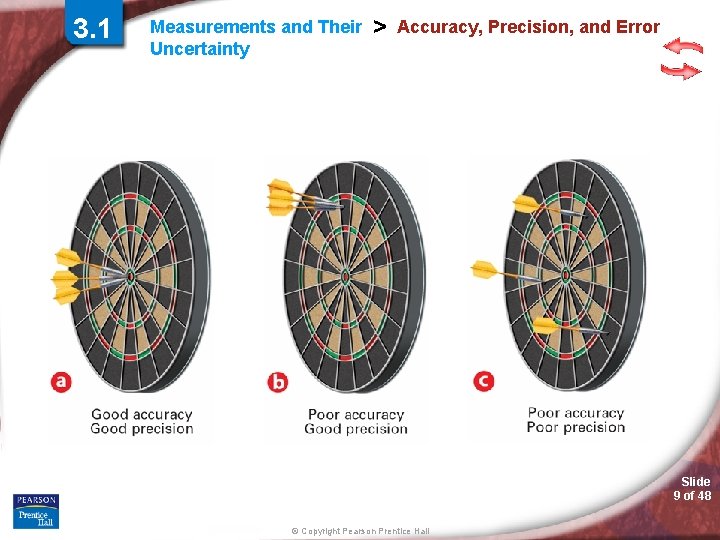
3. 1 Measurements and Their Uncertainty > Accuracy, Precision, and Error How do you evaluate accuracy and precision? Slide 6 of 48 © Copyright Pearson Prentice Hall

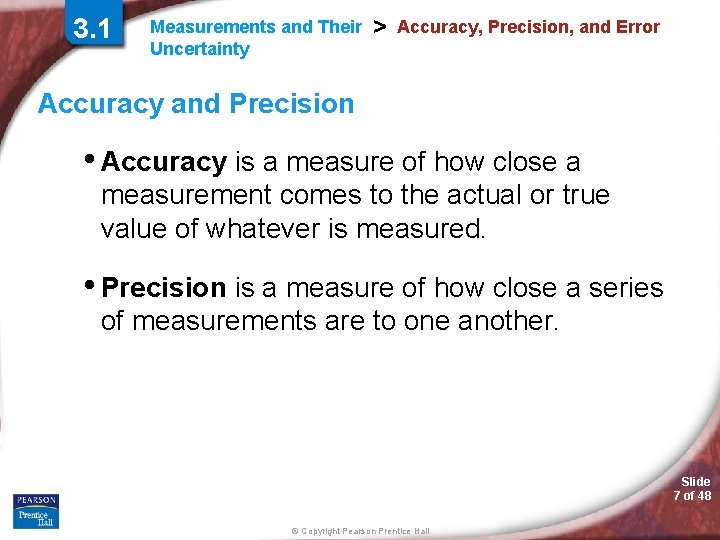
3. 1 Measurements and Their Uncertainty > Accuracy, Precision, and Error Accuracy and Precision • Accuracy is a measure of how close a measurement comes to the actual or true value of whatever is measured. • Precision is a measure of how close a series of measurements are to one another. Slide 7 of 48 © Copyright Pearson Prentice Hall

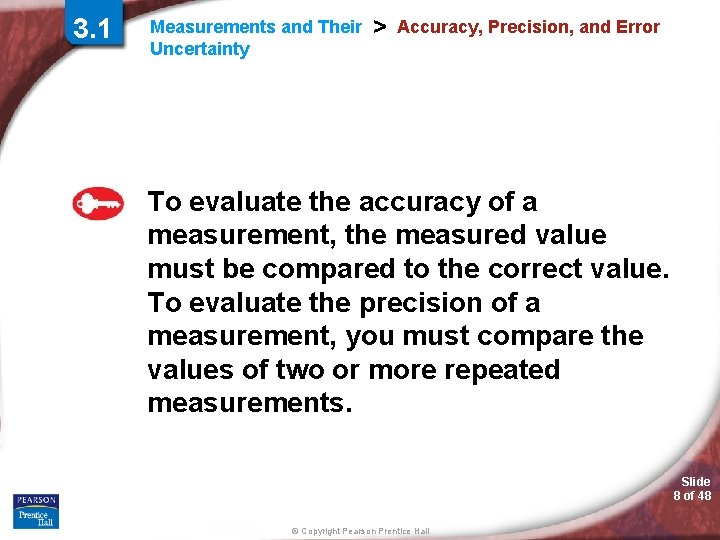
3. 1 Measurements and Their Uncertainty > Accuracy, Precision, and Error To evaluate the accuracy of a measurement, the measured value must be compared to the correct value. To evaluate the precision of a measurement, you must compare the values of two or more repeated measurements. Slide 8 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Accuracy, Precision, and Error Slide 9 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Accuracy, Precision, and Error Determining Error • The accepted value is the correct value based on reliable references. • The experimental value is the value measured in the lab. • The difference between the experimental value and the accepted value is called the error. Slide 10 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Accuracy, Precision, and Error The percent error is the absolute value of the error divided by the accepted value, multiplied by 100%. Slide 11 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Accuracy, Precision, and Error Slide 12 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Accuracy, Precision, and Error Just because a measuring device works, you cannot assume it is accurate. The scale below has not been properly zeroed, so the reading obtained for the person’s weight is inaccurate. Slide 13 of 48 © Copyright Pearson Prentice Hall

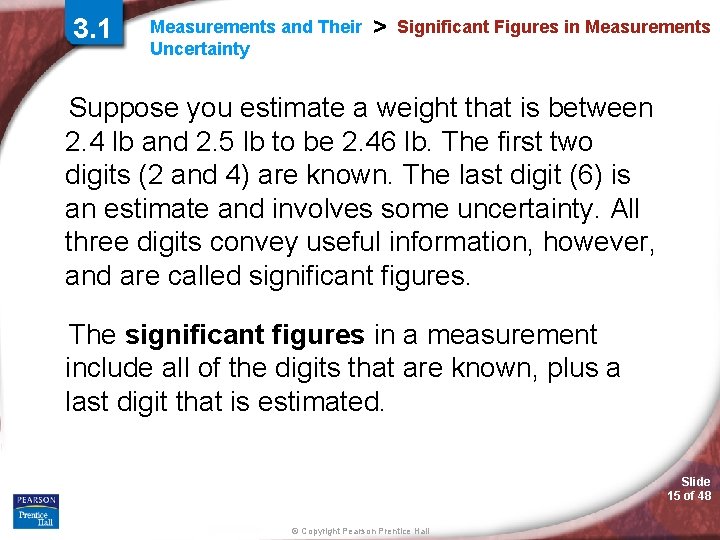
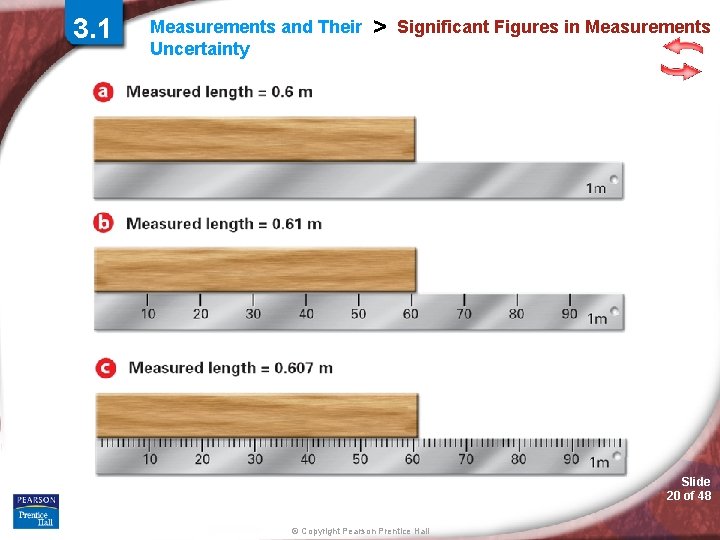
3. 1 Measurements and Their Uncertainty > Significant Figures in Measurements Why must measurements be reported to the correct number of significant figures? Slide 14 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Significant Figures in Measurements Suppose you estimate a weight that is between 2. 4 lb and 2. 5 lb to be 2. 46 lb. The first two digits (2 and 4) are known. The last digit (6) is an estimate and involves some uncertainty. All three digits convey useful information, however, and are called significant figures. The significant figures in a measurement include all of the digits that are known, plus a last digit that is estimated. Slide 15 of 48 © Copyright Pearson Prentice Hall

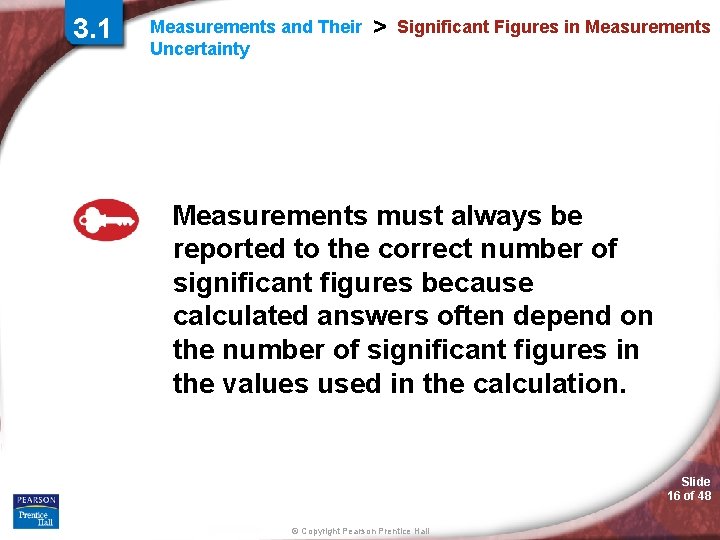
3. 1 Measurements and Their Uncertainty > Significant Figures in Measurements must always be reported to the correct number of significant figures because calculated answers often depend on the number of significant figures in the values used in the calculation. Slide 16 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Significant Figures in Measurements Slide 17 of 48 © Copyright Pearson Prentice Hall

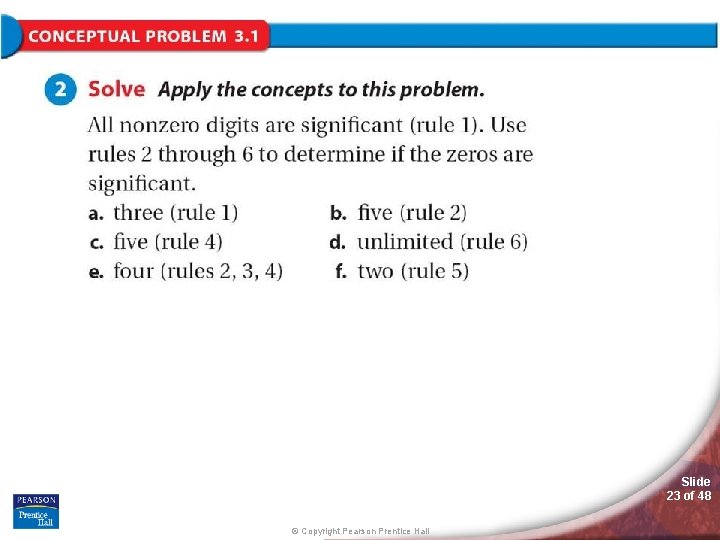
3. 1 Measurements and Their Uncertainty > Significant Figures in Measurements Insert Illustration of a sheet of paper listing the six rules for determining whether a digit in a measured value is significant. Redo the illustration as process art. Each rule should be a separate image. Slide 18 of 48 © Copyright Pearson Prentice Hall

Measurements and Their Uncertainty > Significant Figures in Measurements Animation 2 See how the precision of a calculated result depends on the sensitivity of the measuring instruments. Slide 19 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Significant Figures in Measurements Slide 20 of 48 © Copyright Pearson Prentice Hall

Slide 21 of 48 © Copyright Pearson Prentice Hall

Slide 22 of 48 © Copyright Pearson Prentice Hall

Slide 23 of 48 © Copyright Pearson Prentice Hall

Practice Problems for Conceptual Problem 3. 1 Problem Solving 3. 2 Solve Problem 2 with the help of an interactive guided tutorial. Slide 24 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Significant Figures in Calculations How does the precision of a calculated answer compare to the precision of the measurements used to obtain it? Slide 25 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Significant Figures in Calculations In general, a calculated answer cannot be more precise than the least precise measurement from which it was calculated. The calculated value must be rounded to make it consistent with the measurements from which it was calculated. Slide 26 of 48 © Copyright Pearson Prentice Hall

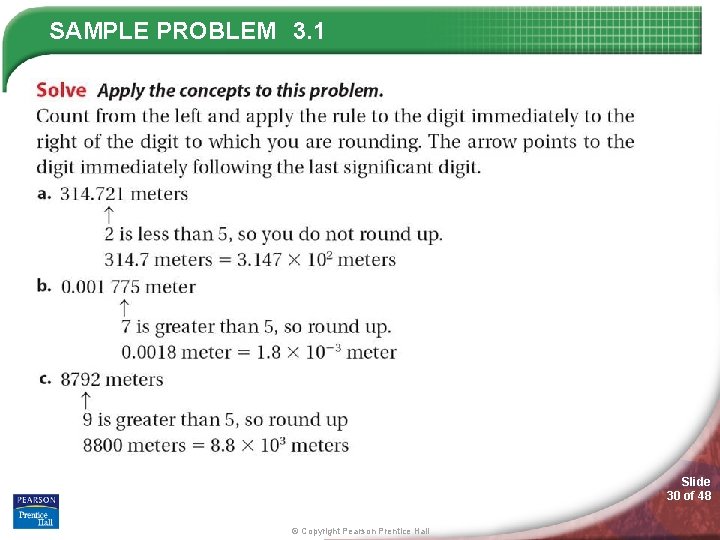
3. 1 Measurements and Their Uncertainty > Significant Figures in Calculations Rounding To round a number, you must first decide how many significant figures your answer should have. The answer depends on the given measurements and on the mathematical process used to arrive at the answer. Slide 27 of 48 © Copyright Pearson Prentice Hall

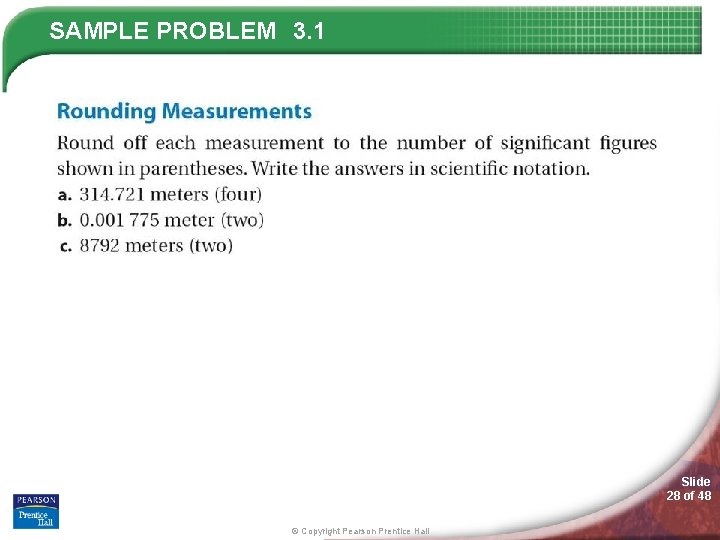
SAMPLE PROBLEM 3. 1 Slide 28 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 1 Slide 29 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 1 Slide 30 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 1 Slide 31 of 48 © Copyright Pearson Prentice Hall

Practice Problems for Sample Problem 3. 1 Problem Solving 3. 3 Solve Problem 3 with the help of an interactive guided tutorial. Slide 32 of 48 © Copyright Pearson Prentice Hall

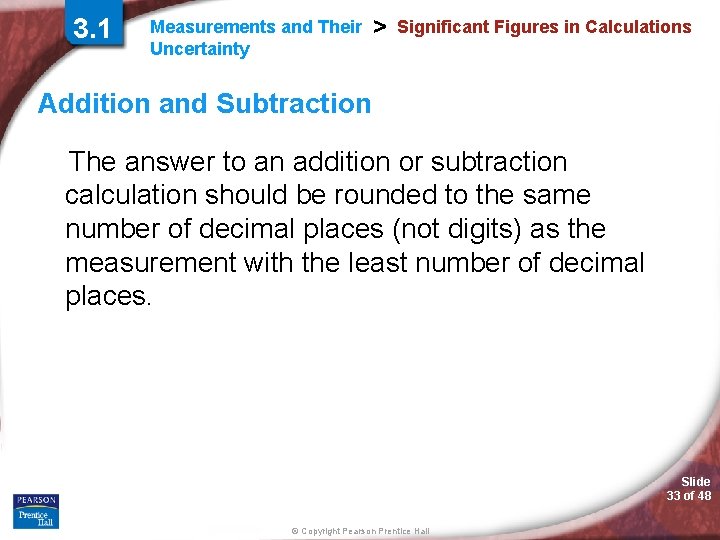
3. 1 Measurements and Their Uncertainty > Significant Figures in Calculations Addition and Subtraction The answer to an addition or subtraction calculation should be rounded to the same number of decimal places (not digits) as the measurement with the least number of decimal places. Slide 33 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 2 Slide 34 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 2 Slide 35 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 2 Slide 36 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 2 Slide 37 of 48 © Copyright Pearson Prentice Hall

Practice Problems for Sample Problem 3. 2 Problem Solving 3. 6 Solve Problem 6 with the help of an interactive guided tutorial. Slide 38 of 48 © Copyright Pearson Prentice Hall

3. 1 Measurements and Their Uncertainty > Significant Figures in Calculations Multiplication and Division • In calculations involving multiplication and division, you need to round the answer to the same number of significant figures as the measurement with the least number of significant figures. • The position of the decimal point has nothing to do with the rounding process when multiplying and dividing measurements. Slide 39 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 3 Slide 40 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 3 Slide 41 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 3 Slide 42 of 48 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 3. 3 Slide 43 of 48 © Copyright Pearson Prentice Hall

Practice Problems for Sample Problem 3. 3 Problem Solving 3. 8 Solve Problem 8 with the help of an interactive guided tutorial. Slide 44 of 48 © Copyright Pearson Prentice Hall

Section Assessment Assess students’ understanding of the concepts in Section 3. 1. Continue to: -or- Launch: Section Quiz Slide 45 of 48 © Copyright Pearson Prentice Hall

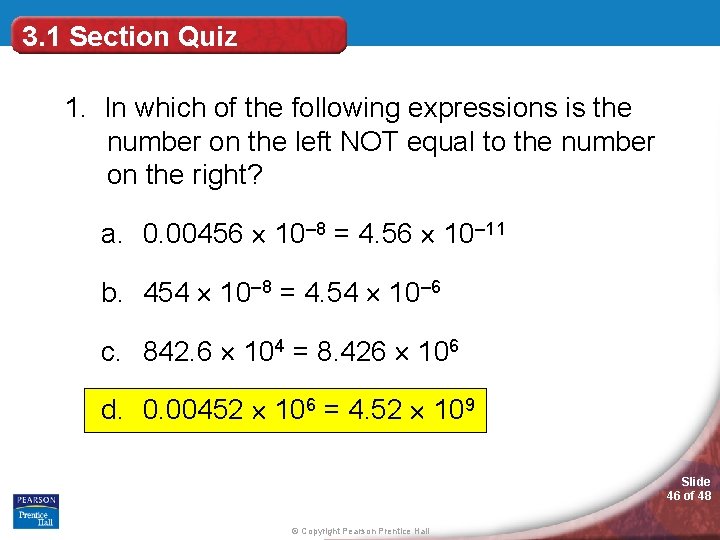
3. 1 Section Quiz 1. In which of the following expressions is the number on the left NOT equal to the number on the right? a. 0. 00456 10– 8 = 4. 56 10– 11 b. 454 10– 8 = 4. 54 10– 6 c. 842. 6 104 = 8. 426 106 d. 0. 00452 106 = 4. 52 109 Slide 46 of 48 © Copyright Pearson Prentice Hall

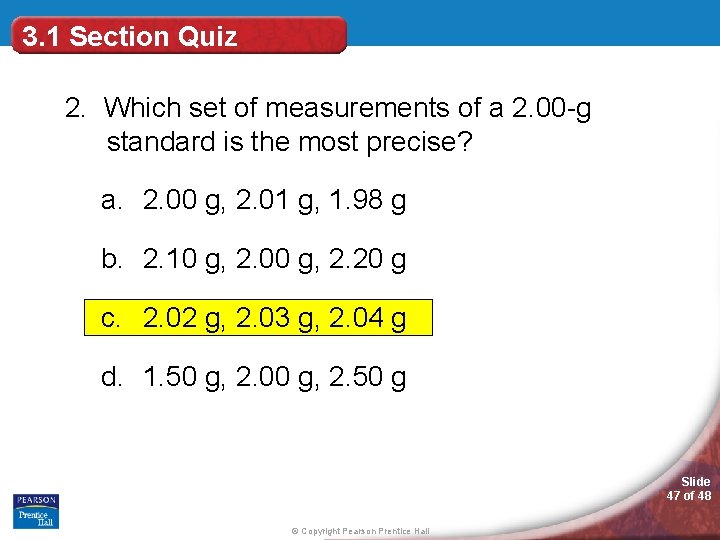
3. 1 Section Quiz 2. Which set of measurements of a 2. 00 -g standard is the most precise? a. 2. 00 g, 2. 01 g, 1. 98 g b. 2. 10 g, 2. 00 g, 2. 20 g c. 2. 02 g, 2. 03 g, 2. 04 g d. 1. 50 g, 2. 00 g, 2. 50 g Slide 47 of 48 © Copyright Pearson Prentice Hall

3. 1 Section Quiz 3. A student reports the volume of a liquid as 0. 0130 L. How many significant figures are in this measurement? a. 2 b. 3 c. 4 d. 5 Slide 48 of 48 © Copyright Pearson Prentice Hall

END OF SHOW
 Measurements and their uncertainty
Measurements and their uncertainty Heel and toe polka dance
Heel and toe polka dance Factor x^2 - 25
Factor x^2 - 25 Measurements equivalents and adjustments
Measurements equivalents and adjustments Chapter 2 measurements and calculations
Chapter 2 measurements and calculations Force and torque measurements
Force and torque measurements Ee8403 measurements and instrumentation
Ee8403 measurements and instrumentation Anthropometric measurement includes vital signs
Anthropometric measurement includes vital signs Sign chapter 37
Sign chapter 37 Segment lengths in circles formulas
Segment lengths in circles formulas Orthostatic vitals positive
Orthostatic vitals positive Vital signs and anthropometric measurements:
Vital signs and anthropometric measurements: Measurement topic
Measurement topic Pharmaceutical measurements
Pharmaceutical measurements Diamtral
Diamtral Metrology and measurements subject code
Metrology and measurements subject code Abbreviation for tablespoon
Abbreviation for tablespoon Measurements and calculations chapter 2 test
Measurements and calculations chapter 2 test Metric system in pharmacy
Metric system in pharmacy Scalar quantity unit
Scalar quantity unit Using and expressing measurements
Using and expressing measurements Measurements and scientific tools lesson 2
Measurements and scientific tools lesson 2 Module 7 weights and measures
Module 7 weights and measures Apothecary conversion chart
Apothecary conversion chart Instrumentation and measurements
Instrumentation and measurements Instrumentation and measurements
Instrumentation and measurements Gdp types
Gdp types Using and expressing measurements
Using and expressing measurements Prologue romeo juliet
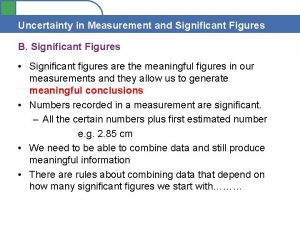
Prologue romeo juliet Uncertainty in measurement and significant digits
Uncertainty in measurement and significant digits What is risk continuum
What is risk continuum Is it real issue with genuine controversy and uncertainty
Is it real issue with genuine controversy and uncertainty Low and high uncertainty avoidance
Low and high uncertainty avoidance Difference between risk and uncertainty
Difference between risk and uncertainty Laray m. barna (1997)
Laray m. barna (1997) Error propagation multiplication
Error propagation multiplication Uncertainty of a protractor
Uncertainty of a protractor Mass society and democracy lesson 2
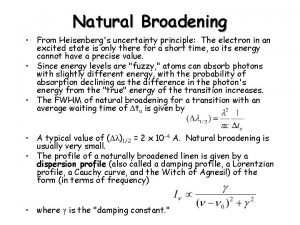
Mass society and democracy lesson 2 Natural broadening
Natural broadening Capital budgeting under risk and uncertainty
Capital budgeting under risk and uncertainty Risk and uncertainty in farm management
Risk and uncertainty in farm management Certainty equivalent
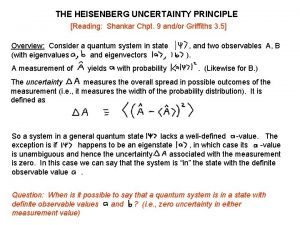
Certainty equivalent State heisenberg uncertainty principle class 11
State heisenberg uncertainty principle class 11 What two measurements are necessary for calculating speed?
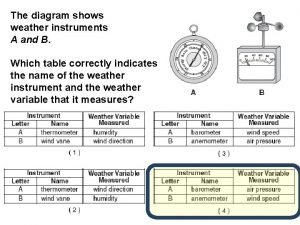
What two measurements are necessary for calculating speed? The diagram below shows weather instruments a and b
The diagram below shows weather instruments a and b Noah's ark measurements
Noah's ark measurements Significant figures rules
Significant figures rules Significant figures in measurements
Significant figures in measurements Statistic vs parameter example
Statistic vs parameter example Qualitative vs quantitative science
Qualitative vs quantitative science