Measurements and graphing Why is it important to





















- Slides: 43

Measurements and graphing Why is it important to know what size shoe you wear? size

Metric Olympics! § Perform the tasks!

Graphing § DRY MIX § Line graphs: § Bar graphs: § Pie graphs

Describe these Objects with Measurements!!!

Look at the pictures…. § Write down what you think should be measured about the object? § What you could use to measure the object? § What units would you measure them in? § What event does it relate to?

BOARD 1

BOARD 2

BOARD 3

BOARD 4

BOARD 5

BOARD 6

BOARD 7

Basic Science Measurement and Metrics

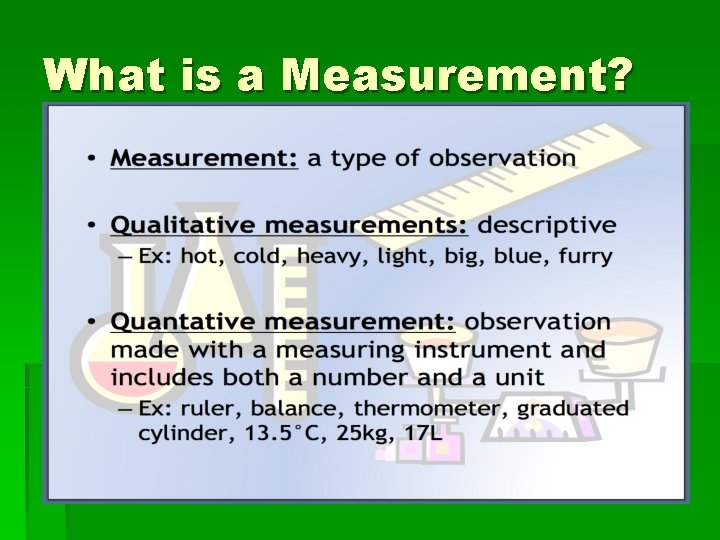
What is a Measurement?

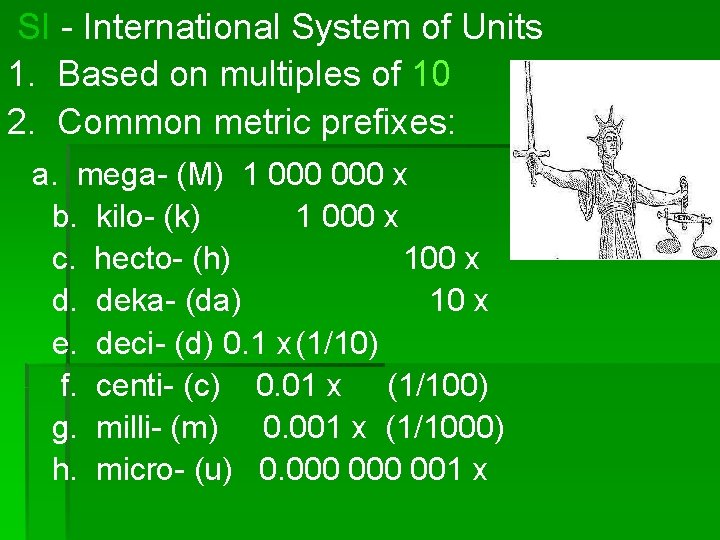
SI - International System of Units 1. Based on multiples of 10 2. Common metric prefixes: a. mega- (M) 1 000 x b. kilo- (k) 1 000 x c. hecto- (h) 100 x d. deka- (da) 10 x e. deci- (d) 0. 1 x(1/10) f. centi- (c) 0. 01 x (1/100) g. milli- (m) 0. 001 x (1/1000) h. micro- (u) 0. 000 001 x

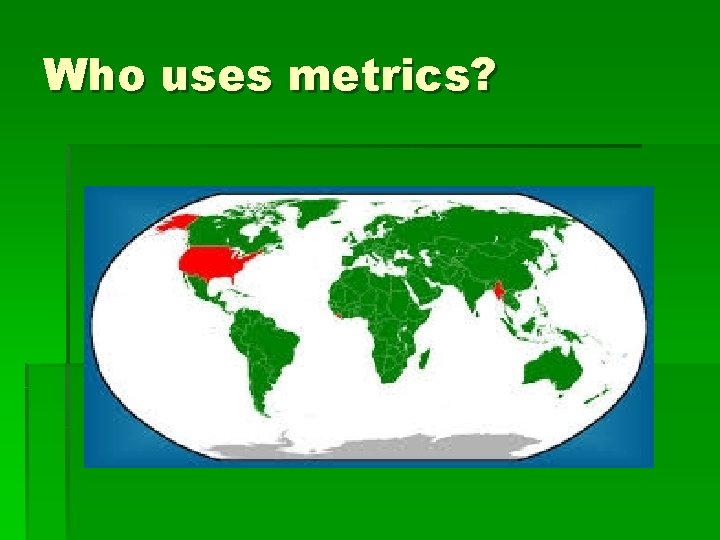
Who uses metrics?

Do the crown!

Practice converting! § Practice worksheet.

“How Stuff Works” § http: //science. howstuffworks. com/why-usnot-on-metric-system. htm

Types of Measurements 1. Length a. The distance from one point to another point. b. Base unit is the meter (m). c. Tool is the metric ruler. 2. Volume a. The amount of space a substance occupies. b. Base unit is the liter (L). c. Tools: metric ruler for regular solids or graduated cylinder for liquids.

3 . Mass a. The amount of matter in a substance. b. Base unit - kilogram (kg). c. Tool is the balance. 4. Weight a. A measure of gravitational force on an object. b. Unit is the newton (N). c. Tool is the scale.

. Time a. How long an event takes to occur. b. Unit is the second (s). c. Tool is the clock (stopwatch). 6. Temperature a. The amount of kinetic energy a substance has. b. SI unit is the Kelvin (K). 5 c. Tool is thermometer.

Amount of object 7. Mol a. How many atoms, molecules, formula units, particles – ANYTHING! b. Basic unit mol c. Tool is math!

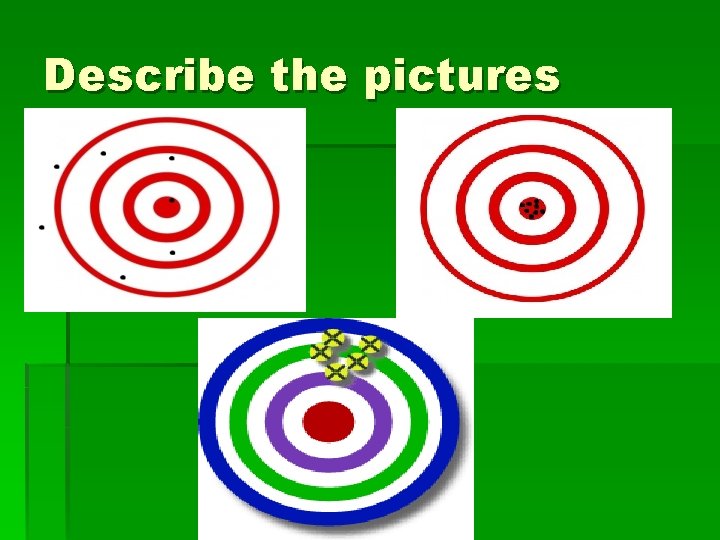
Describe the pictures

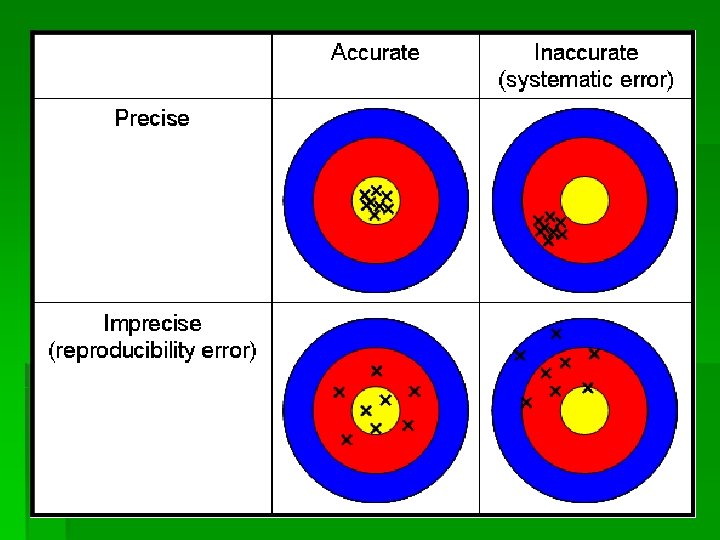
Which is which? § § Which is accurate? Precise? Anything else? Why? § Groups discuss. § Pin the tail

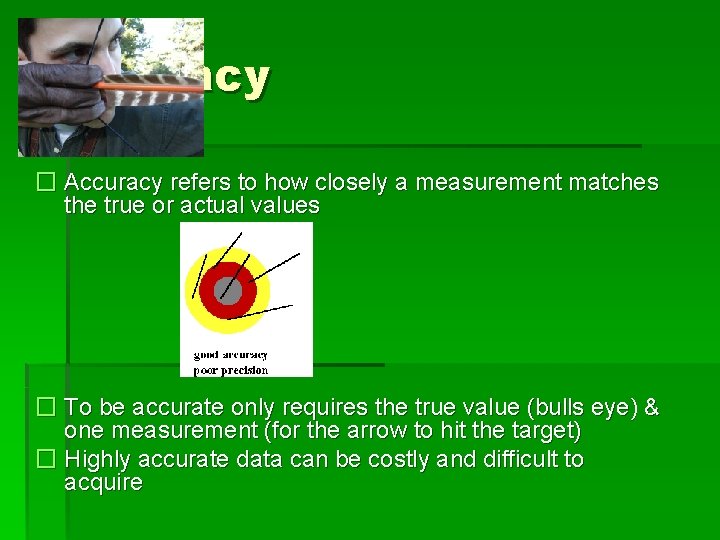
Accuracy � Accuracy refers to how closely a measurement matches the true or actual values � To be accurate only requires the true value (bulls eye) & one measurement (for the arrow to hit the target) � Highly accurate data can be costly and difficult to acquire

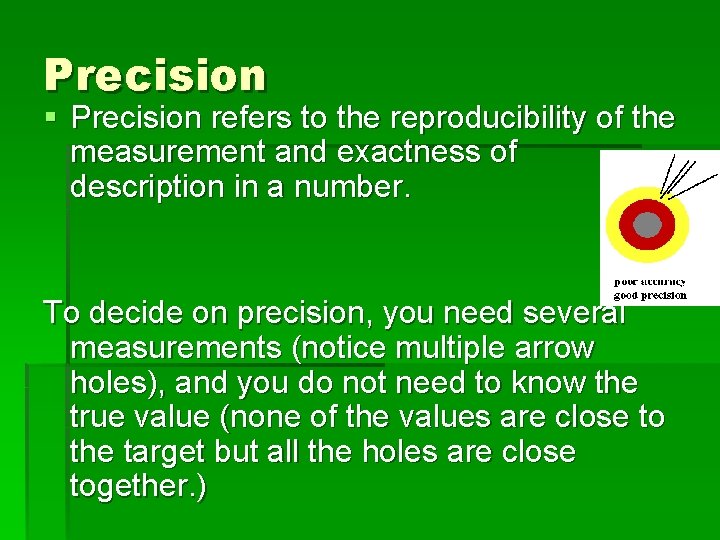
Precision § Precision refers to the reproducibility of the measurement and exactness of description in a number. To decide on precision, you need several measurements (notice multiple arrow holes), and you do not need to know the true value (none of the values are close to the target but all the holes are close together. )

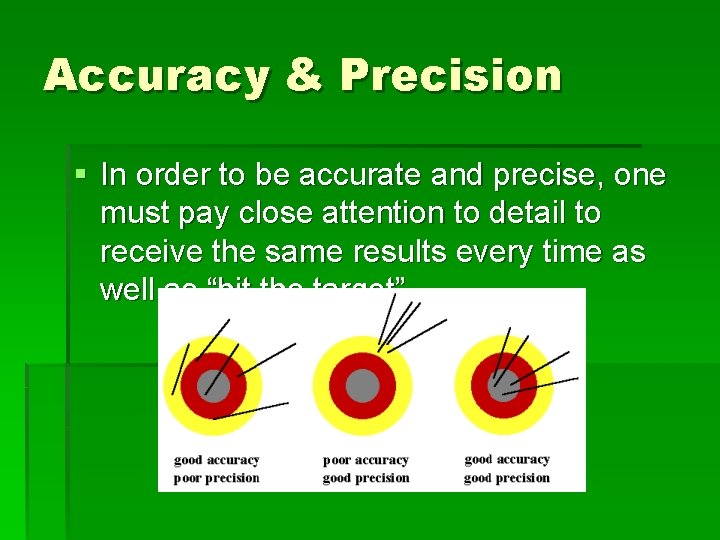
Accuracy & Precision § In order to be accurate and precise, one must pay close attention to detail to receive the same results every time as well as “hit the target”.

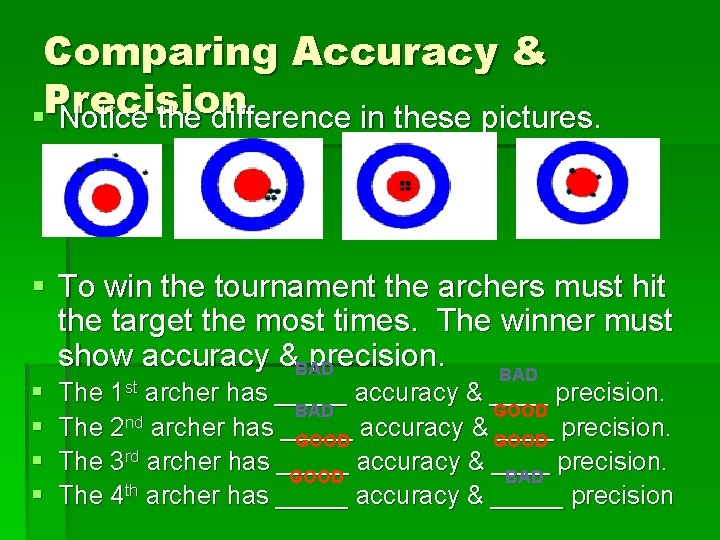
Comparing Accuracy & §Precision Notice the difference in these pictures. § To win the tournament the archers must hit the target the most times. The winner must show accuracy &BAD precision. BAD § § The 1 st archer has _____ accuracy & ____ precision. BAD GOOD nd The 2 archer has _____ accuracy & GOOD ____ precision. GOOD The 3 rd archer has _____ accuracy & ____ precision. GOOD BAD The 4 th archer has _____ accuracy & _____ precision

Example 1 § A sample is known to weigh 3. 182 g. Jane weighed the sample five different times with the resulting data. Which measurement was the most accurate? § 3. 200 g § 3. 180 g § 3. 152 g § 3. 189 g

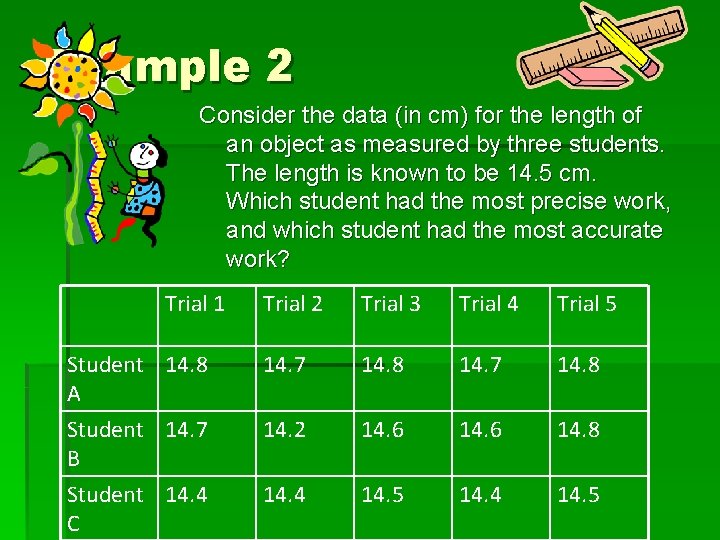
Example 2 Consider the data (in cm) for the length of an object as measured by three students. The length is known to be 14. 5 cm. Which student had the most precise work, and which student had the most accurate work? Trial 1 Student 14. 8 A Student 14. 7 B Student 14. 4 C Trial 2 Trial 3 Trial 4 Trial 5 14. 7 14. 8 14. 2 14. 6 14. 8 14. 4 14. 5

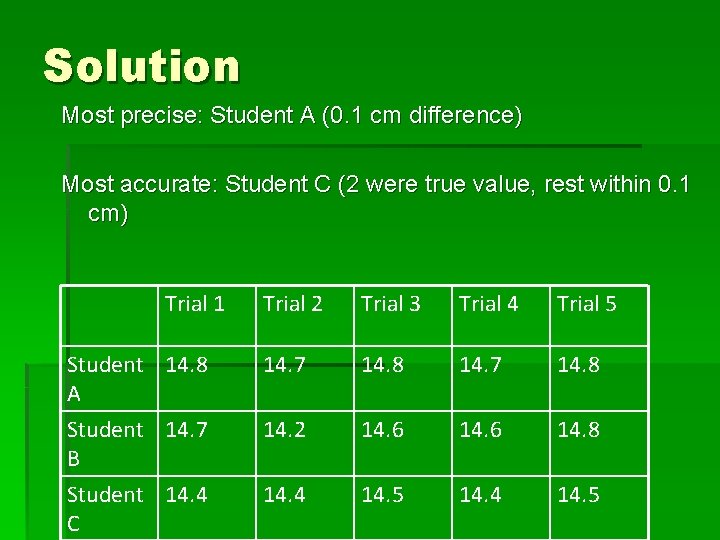
Solution Most precise: Student A (0. 1 cm difference) Most accurate: Student C (2 were true value, rest within 0. 1 cm) Trial 1 Student 14. 8 A Student 14. 7 B Student 14. 4 C Trial 2 Trial 3 Trial 4 Trial 5 14. 7 14. 8 14. 2 14. 6 14. 8 14. 4 14. 5


Significant Figures! § MONEY!!!!! § You won the lotto!!! What would you like to know?

Significant Figures § Why are significant figures necessary? § True accuracy is no better than the measurement obtained by the least precise method. § We use significant digits so we are not exaggerating our precision.

Rules of Significant Digits 1. All digits 1 through 9 are significant. 9. 342 mg = 4 Sig. Digits 233, 124 = 6 sig. digits

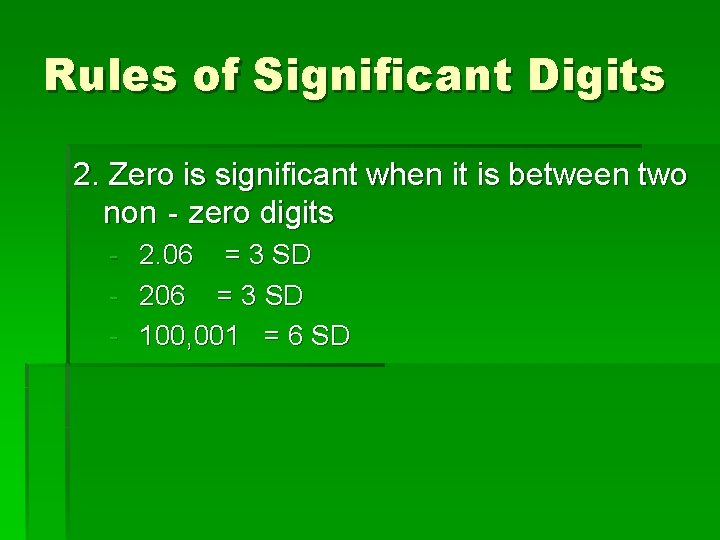
Rules of Significant Digits 2. Zero is significant when it is between two non‐zero digits - 2. 06 = 3 SD - 206 = 3 SD - 100, 001 = 6 SD

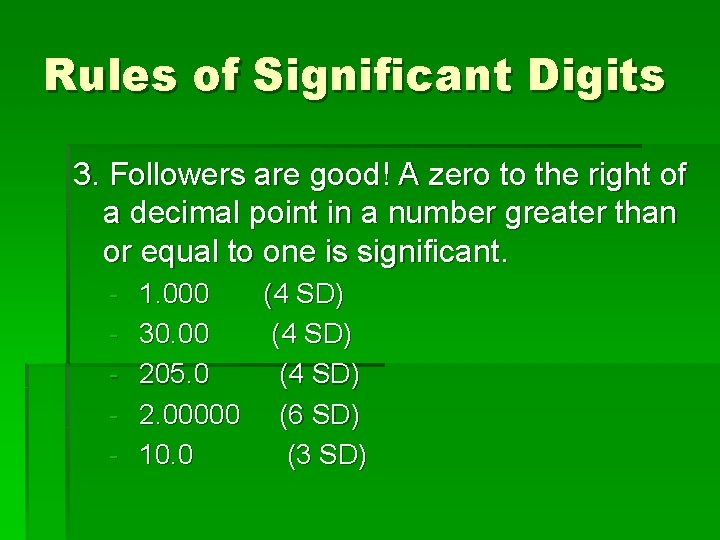
Rules of Significant Digits 3. Followers are good! A zero to the right of a decimal point in a number greater than or equal to one is significant. - 1. 000 (4 SD) 30. 00 (4 SD) 205. 0 (4 SD) 2. 00000 (6 SD) 10. 0 (3 SD)

Rules of Significant Digits 4. Leaders are losers. A zero to the right of a decimal point (in a number less than one) but to the left of nonzero digit is not significant. - 0. 001020 - 0. 00024200 (4 SD) (5 SD)

Rules of Significant Digits 5. No decimal no good. Zeros used only to space the decimal point (placeholders) are not significant. - 1000 - 1010 -78, 000 (1 SD) (3 SD) (2 SD)

What is significant? § In groups use the following list to determine the number of significant figures are recorded in each example. § How did you know?

Summarize § Why are sig figs important?

Review for test! § § § § Lab safety Lab equipment Measurements Accuracy Precision Significant figures Graphing.