Measurements and Conversions Why Measure Measurements give specific















- Slides: 24

Measurements and Conversions

Why Measure? • Measurements give specific information • Different types: - Base Units: System International Units (or Metric) • Standard for many scientific measurements - Derived Units • Combinations of base units • Ex: density or volume

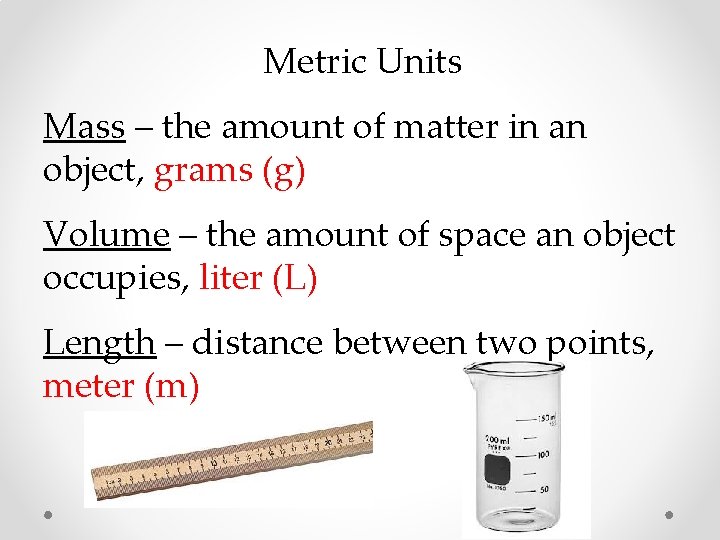
Metric Units Mass – the amount of matter in an object, grams (g) Volume – the amount of space an object occupies, liter (L) Length – distance between two points, meter (m)

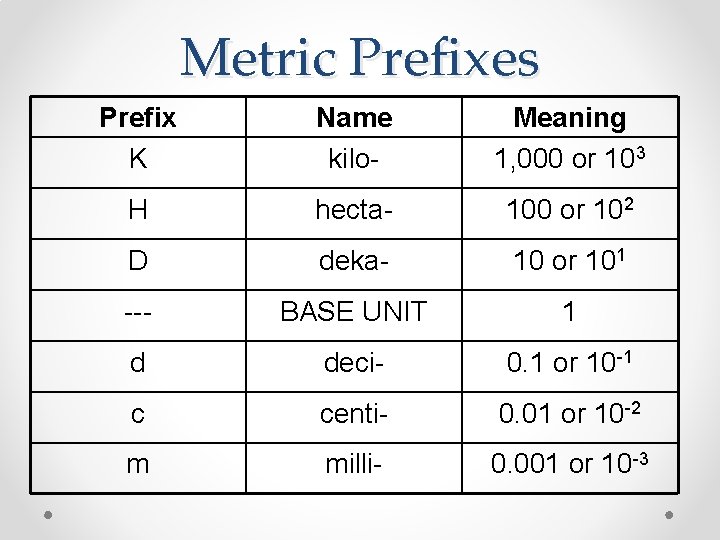
Metric Prefixes Prefix K Name kilo- Meaning 1, 000 or 103 H hecta- 100 or 102 D deka- 10 or 101 --- BASE UNIT 1 d deci- 0. 1 or 10 -1 c centi- 0. 01 or 10 -2 m milli- 0. 001 or 10 -3

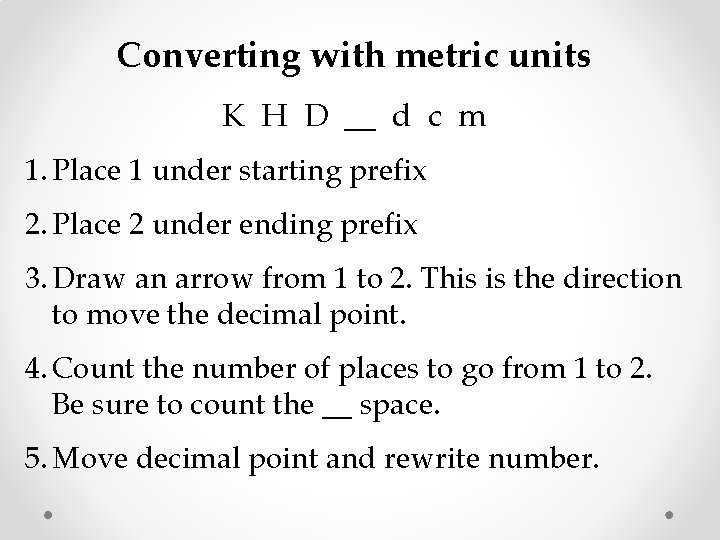
Converting with metric units K H D __ d c m 1. Place 1 under starting prefix 2. Place 2 under ending prefix 3. Draw an arrow from 1 to 2. This is the direction to move the decimal point. 4. Count the number of places to go from 1 to 2. Be sure to count the __ space. 5. Move decimal point and rewrite number.

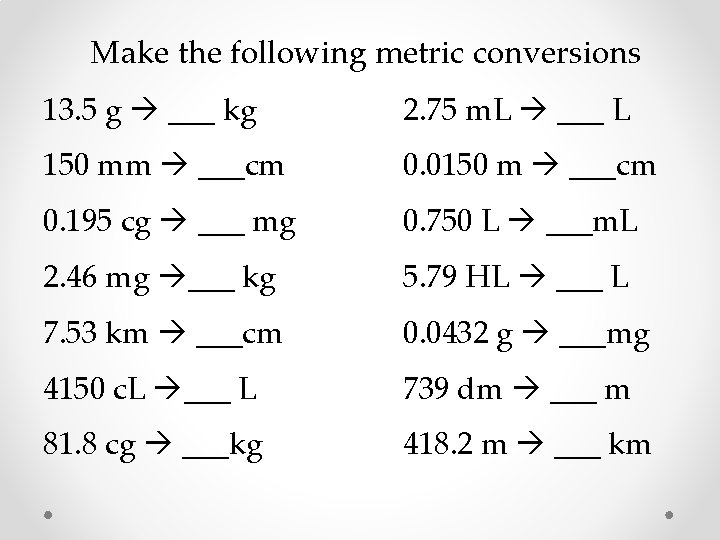
Make the following metric conversions 13. 5 g ___ kg 2. 75 m. L ___ L 150 mm ___cm 0. 0150 m ___cm 0. 195 cg ___ mg 0. 750 L ___m. L 2. 46 mg ___ kg 5. 79 HL ___ L 7. 53 km ___cm 0. 0432 g ___mg 4150 c. L ___ L 739 dm ___ m 81. 8 cg ___kg 418. 2 m ___ km

Scientific Notation – a way of showing very large or small numbers. 4. 7 x 103 4. 7 E 3 4. 7 exp 3 4. 7 x 10 = 4, 700 • Only significant numbers remain • The “number out front” is only allowed to have one nonzero digit to the left of the decimal point.

Converting TO Scientific Notation 1. Place the decimal after the first digit 2. Count from the original decimal place to the new location. o That number will become the exponent. 3. If you counted: o o To the left (starting # > 1) then + exponent To the right (starting #< 1) then - exponent 4. Write “new” number x 10 exponent 5. Do not write any placeholding zeros o Non-significant numbers

Converting FROM Scientific Notation 1. The exponent or power tells how many places the decimal point will be moved. 2. If the exponent is positive, the decimal point moves to the right. - The number will be > 1 3. If the exponent is negative, the decimal point moves to the left. - The number will be < 1

Warm-Up • Convert the following: 1. 2. 3. 41, 300 L to k. L 13, 200 kg to g 72 cm to mm 1. 2. 3. 41. 3 k. L 13, 200, 000 g 720 mm

Warm-Up • Express is Scientific Notation: 1. 2. 3. 1600 0. 0053 134. 8 1. 6 x 103 5. 3 x 10 -3 1. 348 x 102

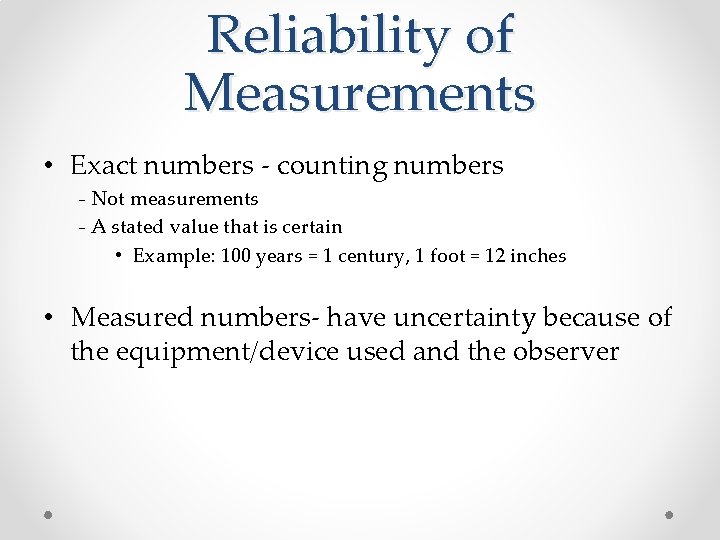
Reliability of Measurements • Exact numbers - counting numbers - Not measurements - A stated value that is certain • Example: 100 years = 1 century, 1 foot = 12 inches • Measured numbers- have uncertainty because of the equipment/device used and the observer

Accuracy and Precision • Accuracy - How close the measured value is to the accepted value • Precision - How close a series of measurements are to each other

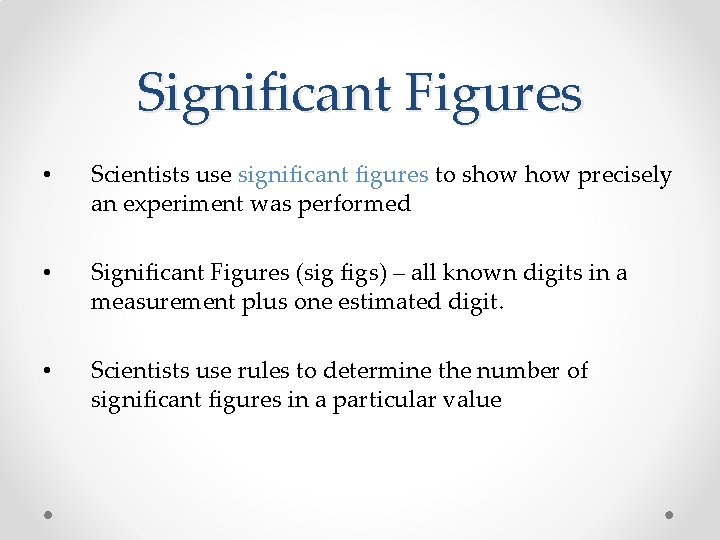
Significant Figures • Scientists use significant figures to show precisely an experiment was performed • Significant Figures (sig figs) – all known digits in a measurement plus one estimated digit. • Scientists use rules to determine the number of significant figures in a particular value

When to use Sig Fig • When something is MEASURED • Not when something is COUNTED

Coast to Coast Sig Figs Atlantic/Pacific Rule: • If a decimal point is absent, count from the Atlantic (right) side starting with the first nonzero digit. • If a decimal point is present, count from the Pacific (left) side starting with the first nonzero digit

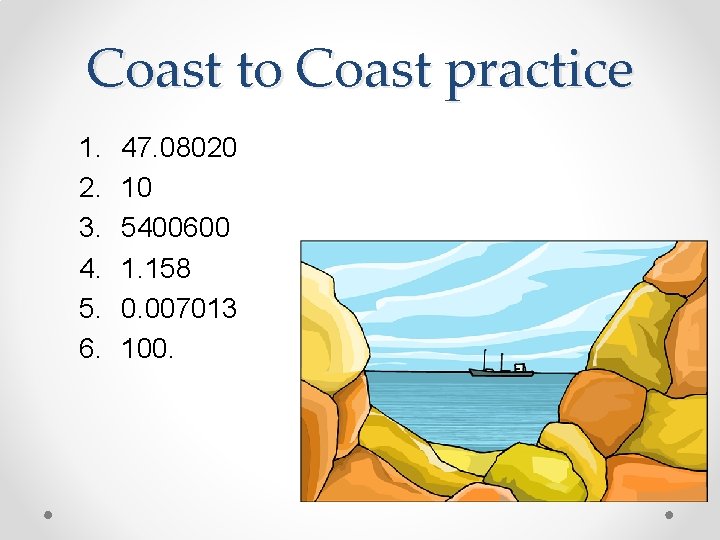
Coast to Coast practice 1. 2. 3. 4. 5. 6. 47. 08020 10 5400600 1. 158 0. 007013 100.

Rounding numbers in calculations • Add/subtract: the answer will have the same number of places past the decimal point as the measurement with the fewest places past the decimal point. • Multiply/divide: the answer will have the same number of sig figs as the measurement with the fewest number of sig figs. • Rounding off numbers: 0 -4 drop, 5 -9 add 1 • Defined or exact numbers have an infinite number of significant figures.

WHEN to round off significant figures • When the rules for rounding change - i. e. going from addition division or multiplication addition • At the end of the problem, and you are giving the final answer

Conversions that you are responsible for knowing… • • 12 in = 1 ft 3 ft = 1 yd 5280 ft = 1 mile 60 sec = 1 min 60 min = 1 hr 24 hr = 1 day 7 day = 1 wk 365. 25 days = 1 yr • • • 16 oz = 1 lb 2000 lb = 1 ton 8 oz = 1 cup 2 cups = 1 pint 2 pints = 1 quart 4 quarts = 1 gallon

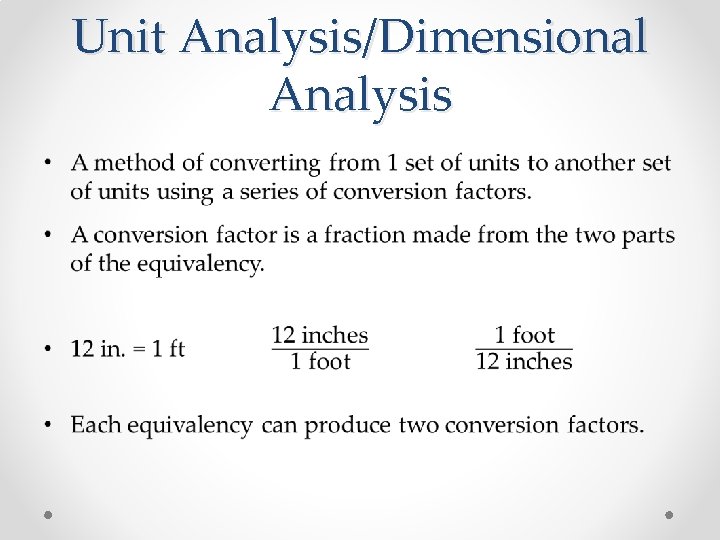
Unit Analysis/Dimensional Analysis •

Convert 22 inches to feet •

Unit Analysis Practice • 5. 75 min months • 135 km cm • 17. 5 oz gal • 1. 5 mile inch • 145 mg hg • 135 m. L L

• 5. 75 min month 5. 75 min_ 1 hr 1 day 60 min 24 hr 1 yr 12 mos = 1. 31 x 104 months 365. 25 day 1 yr • 135 km cm 135 km 1000 m 1 km 1 cm = 13, 500, 000 cm 0. 01 m • 17. 5 oz gal 17. 5 oz 1 qt 1 gal = 0. 138 gal 32 oz 4 qt • 1. 5 mile inch 1. 5 mile 5280 ft 12 in = 1 mile 1 ft 95, 000 in
 Hey hey bye bye
Hey hey bye bye Air temperature
Air temperature What is the main idea of give me liberty or give me death
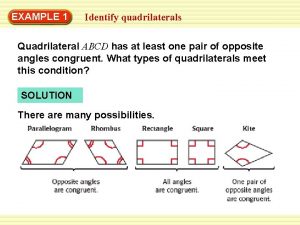
What is the main idea of give me liberty or give me death What is the most specific name for quadrilateral pqrs
What is the most specific name for quadrilateral pqrs Is measure for measure a comedy
Is measure for measure a comedy Calculate weight using specific gravity and volume
Calculate weight using specific gravity and volume Specific weight
Specific weight Don't ask why why why
Don't ask why why why What is your favourite tv show?
What is your favourite tv show? Touching spirit bear study guide
Touching spirit bear study guide Why we must save dying tongues
Why we must save dying tongues How does claudius manipulate laertes
How does claudius manipulate laertes How much land does a man need
How much land does a man need Palm of deborah
Palm of deborah 2-6 practice ratios rates and conversions
2-6 practice ratios rates and conversions Conversions and calculations chapter 6
Conversions and calculations chapter 6 Ratio rates and conversions
Ratio rates and conversions Scientific notation and metric conversions
Scientific notation and metric conversions Thermochemistry conversions
Thermochemistry conversions Energy forms and energy conversions
Energy forms and energy conversions Elementary name and address conversions
Elementary name and address conversions Molecules to moles
Molecules to moles Metric conversions ladder method
Metric conversions ladder method Try these conversions using the ladder method
Try these conversions using the ladder method Measurement maths literacy grade 12
Measurement maths literacy grade 12