Measurement Unit Accuracy vs Precision A Look at








- Slides: 17

Measurement Unit Accuracy vs Precision

A Look at the Vocabulary • The terms “Accuracy” • But, in the Science and “Precision” are realm, we have very often used different definitions for interchangeably within the two terms. the English language • Complicating the (but outside of the discussion a bit is the Science world). fact that Chemistry actually has two different uses for the term “Accuracy”.

Accuracy – 1 st Definition • Defined as how close an experimental measurement is to an “accepted value”. • By “accepted value”, we mean theoretical correct answer.

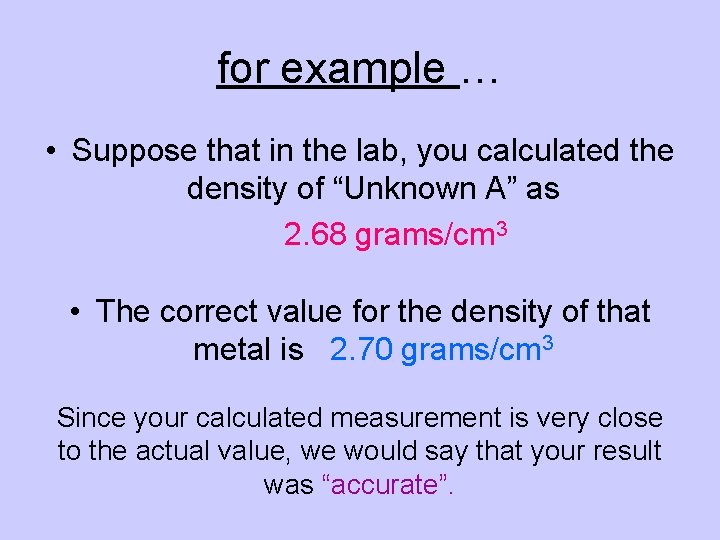
for example … • Suppose that in the lab, you calculated the density of “Unknown A” as 2. 68 grams/cm 3 • The correct value for the density of that metal is 2. 70 grams/cm 3 Since your calculated measurement is very close to the actual value, we would say that your result was “accurate”.

Accuracy – Definition #2 • This definition is used to describe the possible accuracy that one may measure with a given piece of laboratory equipment. • This description will typically report to what decimal place a given measurement can be reported.

Determining This Accuracy • The “rule of thumb” that is used is that we will always imagine there to be 10 lines between the smallest lines on the device. • The decimal place created by those imaginary lines establishes the possible accuracy of the measuring device.

Now what about Precision? • Precision refers to whether a SET OF MEASUREMENTS are CLOSE IN VALUE to EACH OTHER. • It is important to note that the measurements in question may or may not be accurate. • All that you are doing here is comparing a set of measurements to each other.

Consider the visuals on the following slides…

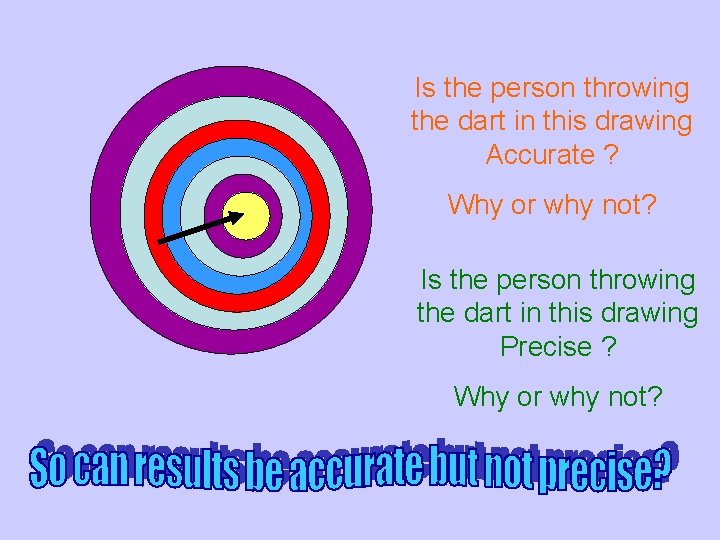
Is the person throwing the dart in this drawing Accurate ? Why or why not? Is the person throwing the dart in this drawing Precise ? Why or why not?

Are the results represented here Accurate ? Why or why not? Are the results represented here Precise ? Why or why not?

So what about the accuracy and precision of these results?

The Determination of Experimental Error

The Two Quantities to Know: Experimental Value: This is the result that is determined by YOU. You actually measured it…. You had your hands on the equipment to determine its value.

Theoretical Value This is the correct answer. It is not typically based on measurements. You can usually find it in a book of “correct answers”.

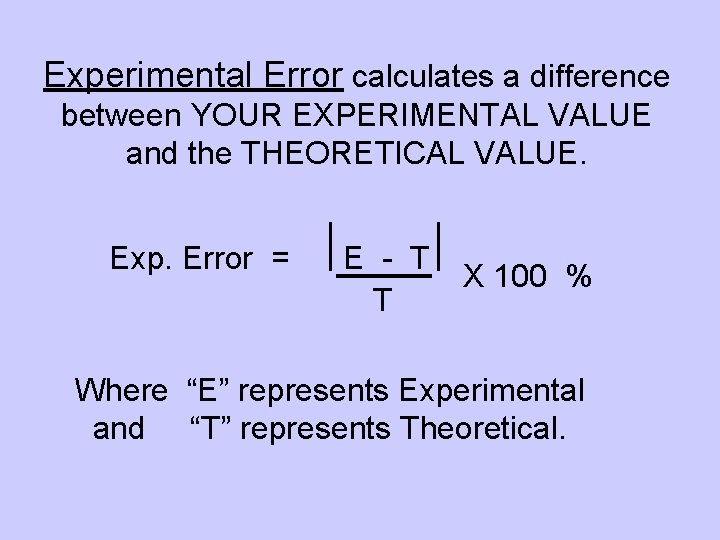
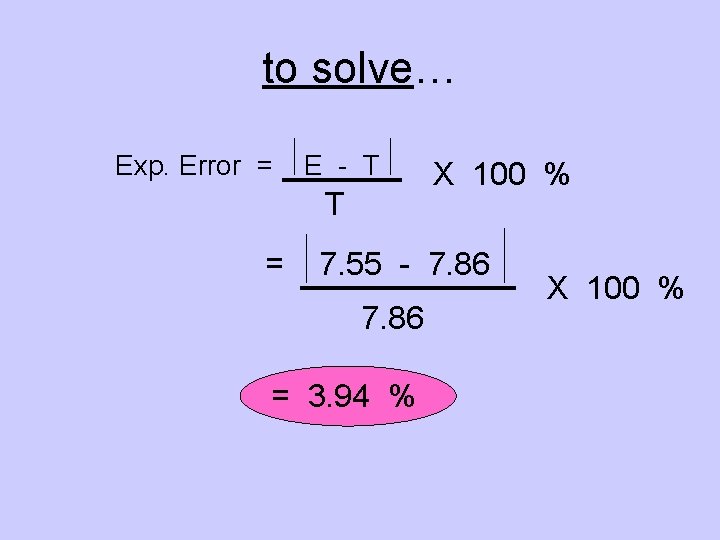
Experimental Error calculates a difference between YOUR EXPERIMENTAL VALUE and the THEORETICAL VALUE. Exp. Error = E - T T X 100 % Where “E” represents Experimental and “T” represents Theoretical.

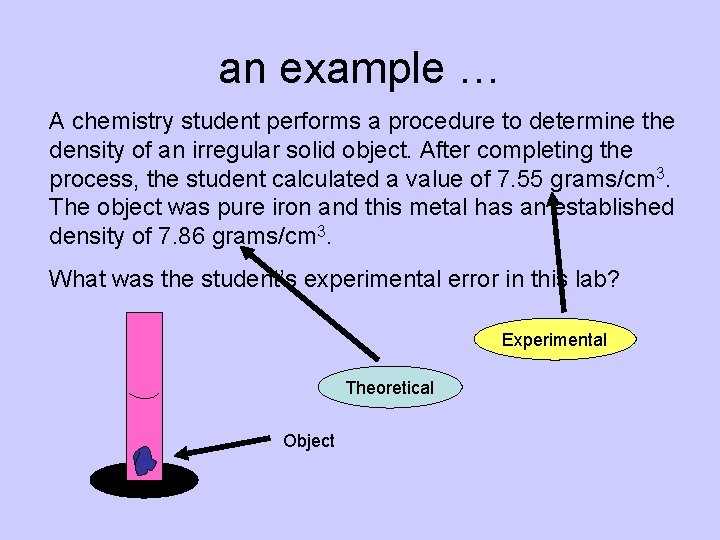
an example … A chemistry student performs a procedure to determine the density of an irregular solid object. After completing the process, the student calculated a value of 7. 55 grams/cm 3. The object was pure iron and this metal has an established density of 7. 86 grams/cm 3. What was the student’s experimental error in this lab? Experimental Theoretical Object

to solve… Exp. Error = E - T T = X 100 % 7. 55 - 7. 86 = 3. 94 % X 100 %