Measurement The Metric System A SI Prefix Conversions






















- Slides: 28

Measurement – The Metric System

A. SI Prefix Conversions 1. Find the difference between the exponents of the two prefixes. 2. Move the decimal that many places. To the left or right?

A. SI Prefix Conversions 532 m NUMBER UNIT 0. 532 km = _______ = NUMBER UNIT

A. SI Prefix Conversions move right move left Prefix mega- Symbol M Factor 106 kilo- k 103 BASE UNIT --- 100 deci- d 10 -1 centi- c 10 -2 milli- m 10 -3 micro- 10 -6 nano- n 10 -9 pico- p 10 -12

A. SI Prefix Conversions 1) 20 cm = 2) 0. 032 L = 3) 45 m = 4) 805 dm = 0. 2 _______ m 32 _______ m. L 45, 000 _______ nm 0. 0805 _______ km

Scientific Notation 65, 000 kg 6. 5 × 104 kg n Converting into Sci. Notation: u u Move decimal until there’s 1 digit to its left. Places moved = exponent. Large # (>1) positive exponent Small # (<1) negative exponent

Scientific Notation Practice Problems 7. 2, 400, 000 g 2. 4 106 g 8. 0. 00256 kg 2. 56 10 -3 kg 9. 7 10 -5 km 0. 00007 km 10. 6. 2 104 mm 62, 000 mm

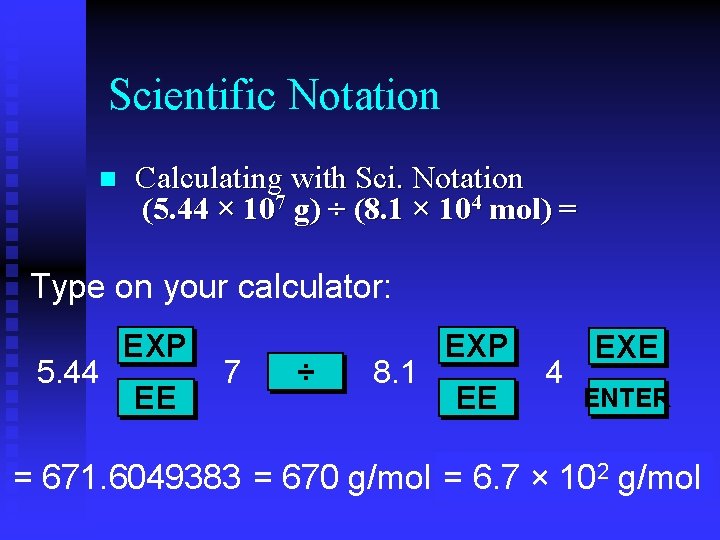
Scientific Notation n Calculating with Sci. Notation (5. 44 × 107 g) ÷ (8. 1 × 104 mol) = Type on your calculator: 5. 44 EXP EE 7 ÷ 8. 1 EXP EE 4 EXE ENTER = 671. 6049383 = 670 g/mol = 6. 7 × 102 g/mol

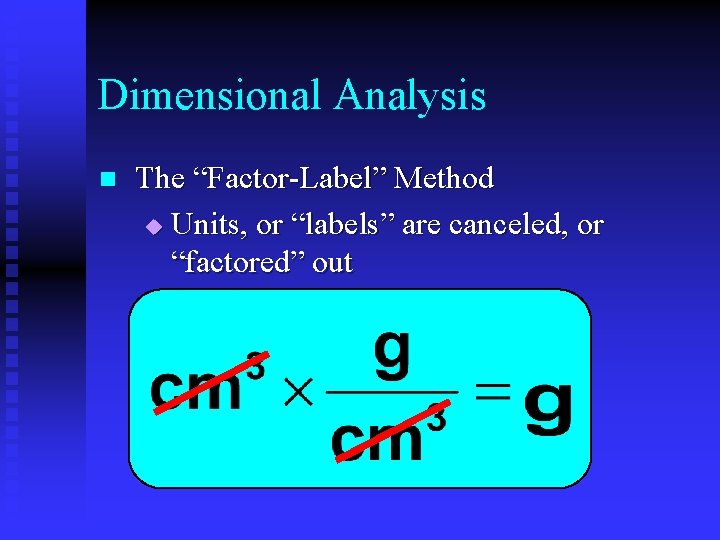
Dimensional Analysis n The “Factor-Label” Method u Units, or “labels” are canceled, or “factored” out

Dimensional Analysis n Steps: 1. Identify starting & ending units. 2. Line up conversion factors so units cancel. 3. Multiply all top numbers & divide by each bottom number. 4. Check units & answer.

Dimensional Analysis n qt How many milliliters are in 1. 00 quart of milk? m. L 1. 00 qt 1 L 1000 m. L 1. 057 qt 1 L = 946 m. L

Dimensional Analysis n lb 1. 5 lb You have 1. 5 pounds of gold. Find its volume in cm 3 if the density of gold is 19. 3 g/cm 3. cm 3 1 kg 1000 g 1 cm 3 2. 2 lb 1 kg 19. 3 g = 35 cm 3

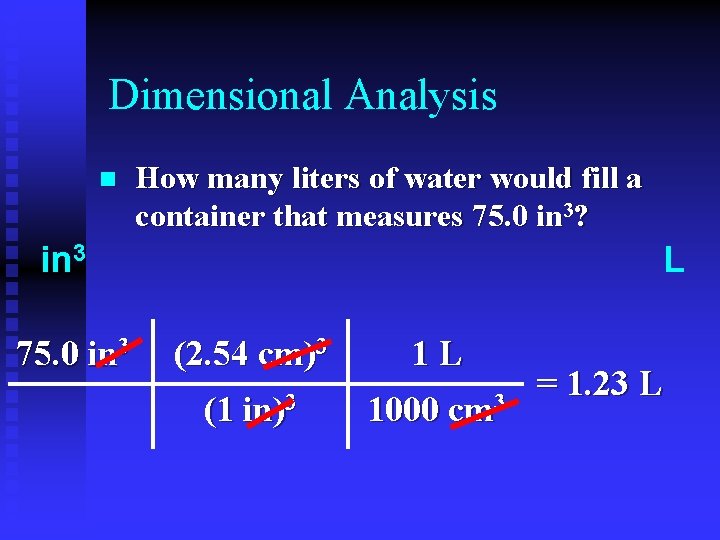
Dimensional Analysis n How many liters of water would fill a container that measures 75. 0 in 3? in 3 75. 0 in 3 L (2. 54 cm)3 1 L (1 in)3 1000 cm 3 = 1. 23 L

Dimensional Analysis cm 5) Your European hairdresser wants to cut your hair 8. 0 cm shorter. How many inches will he be cutting off? in 8. 0 cm 1 in 2. 54 cm = 3. 2 in

Dimensional Analysis cm 6) Taft football needs 550 cm for a 1 st down. How many yards is this? 550 cm 1 in 1 ft 1 yd 2. 54 cm 12 in 3 ft = 6. 0 yd yd

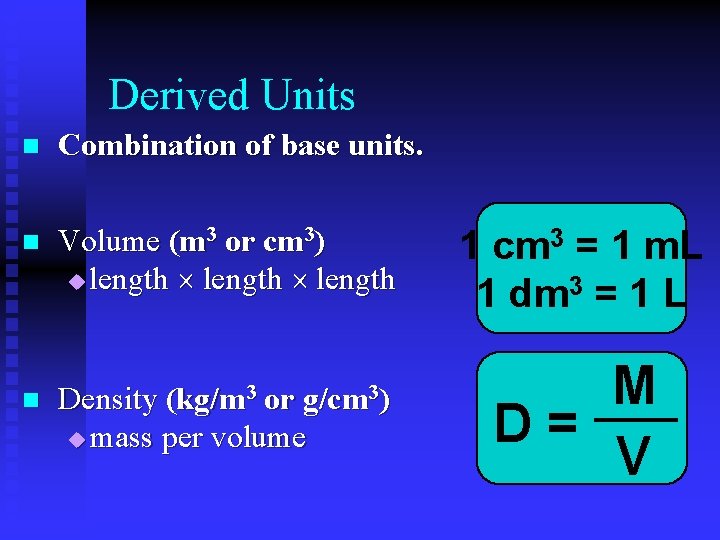
Derived Units n Combination of base units. n Volume (m 3 or cm 3) u length 1 cm 3 = 1 m. L 1 dm 3 = 1 L n Density (kg/m 3 or g/cm 3) u mass per volume M D= V

Density n An object has a volume of 825 cm 3 and a density of 13. 6 g/cm 3. Find its mass. GIVEN: WORK: V = 825 cm 3 D = 13. 6 g/cm 3 M=? M = DV M = (13. 6 g/cm 3)(825 cm 3) M = 11, 200 g

Density n A liquid has a density of 0. 87 g/m. L. What volume is occupied by 25 g of the liquid? GIVEN: WORK: D = 0. 87 g/m. L V=? M = 25 g V=M D V= 25 g 0. 87 g/m. L V = 29 m. L

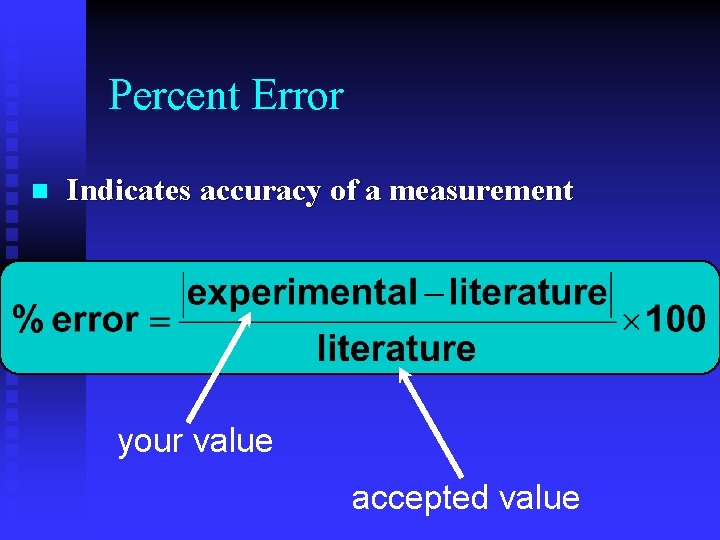
Percent Error n Indicates accuracy of a measurement your value accepted value

Percent Error n A student determines the density of a substance to be 1. 40 g/m. L. Find the % error if the accepted value of the density is 1. 36 g/m. L. % error = 2. 9 %

ACCURACY Accuracy can be defined as how close a number is to what it should be. n Accuracy is determined by comparing a number to a known or accepted value. n

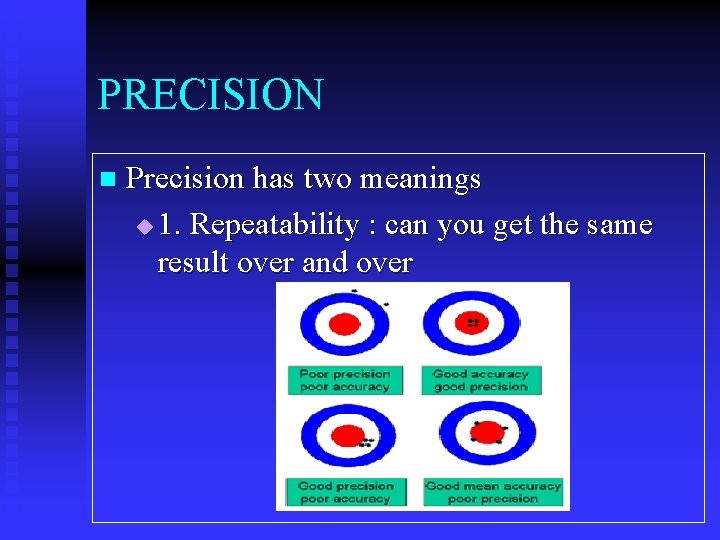
PRECISION n Precision has two meanings u 1. Repeatability : can you get the same result over and over

n Precision can also mean u The number of decimal places assigned to the measured number (The more decimal places, the more precise the measurement)

Example 1: n How old are you? u I am 16 years old u I am 15 years and 8 months old u I am 15 years, 8 months, and 5 days old u I am 15 years, 8 months, 5 days, and 10 hours old

Accuracy vs. Precision for Example 1 Each of these statements is more accurate and more precise than the one before it. n Statement two is more accurate and more precise that statement one. n Statement three is more accurate and more precise than statement two. n

Example 2: How long is a piece of string? u Johnny measures the string at 2. 63 cm. u Using the same ruler, Fred measures the string at 1. 98 cm. u Who is most precise? u Who is most accurate? n The actual measurement is 2. 65 cm. n

Accuracy vs. Precision for Example 2 Johnny is fairly accurate and also very precise. n Fred is very precise, however, he is not very accurate. His lack of accuracy is due to using the ruler incorrectly. n

ACCURACY/PRECISION You can tell the precision of a number simply by looking at it. The number of decimal places gives the precision. n Accuracy on the other hand, depends on comparing a number to a known value. Therefore, you cannot simply look at a number and tell if it is accurate n