Measurement Schmeasurement There is No Such Thing as






- Slides: 17

Measurement Schmeasurement

There is No Such Thing as a Perfect Measurement… The quality of a measurement depends upon: • The precision of the instrument(s) used. • The accuracy of an instrument. • The ability of the instrument user.

Accuracy is the extent to which a reported measurement approaches the true value of the quantity measured. Do we always know the true value? How can we improve upon our accuracy?

Precision is the degree of exactness or refinement of a measurement, and is based on the precision of the measuring instrument.

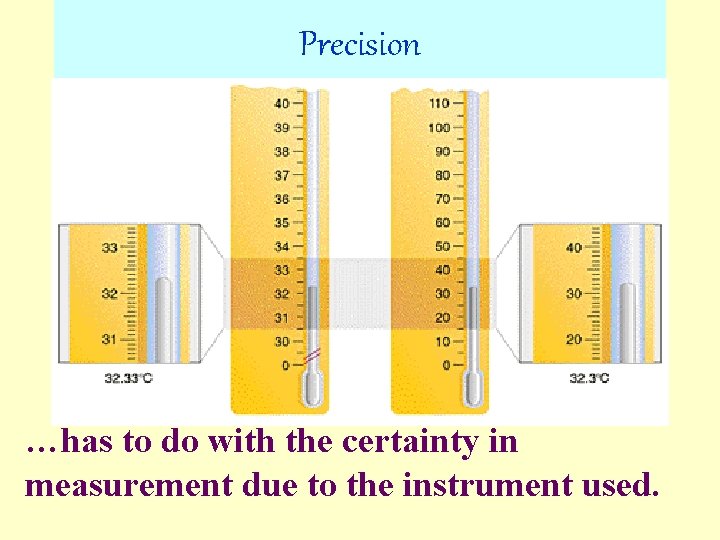
Precision …has to do with the certainty in measurement due to the instrument used.

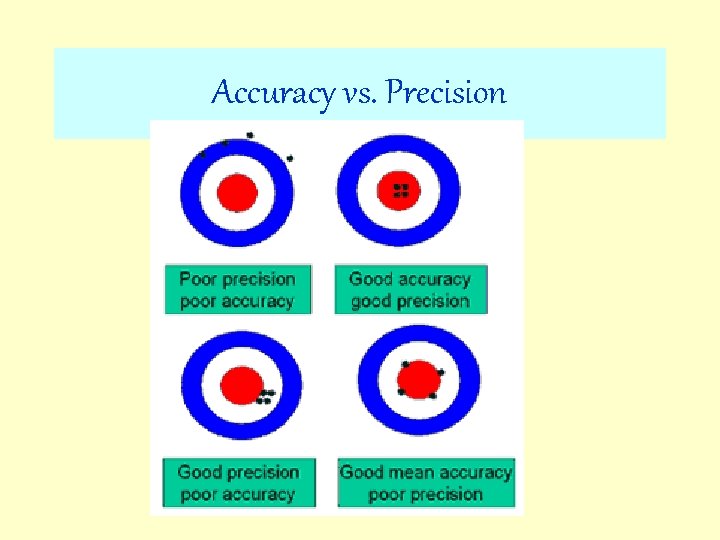
Accuracy vs. Precision Accuracy is telling the truth. . . Precision is telling the same story over and over again. -Yiding Wang

Accuracy vs. Precision

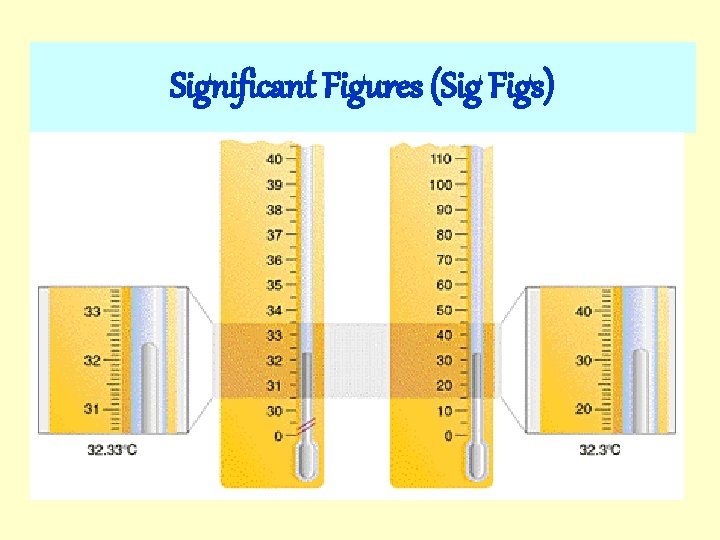
Significant Figures (Sig Figs)

Significant Figures When physical quantities are measured, the measured values are known only to within the limits of the experimental uncertainty. You cannot claim levels of precision that the measuring device you used cannot discern. Significant figures are used to communicate the certainty of a measurement.

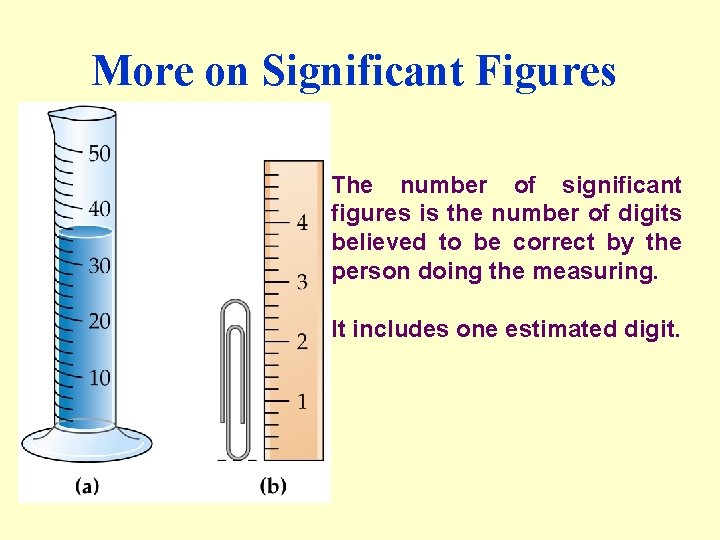
More on Significant Figures The number of significant figures is the number of digits believed to be correct by the person doing the measuring. It includes one estimated digit.

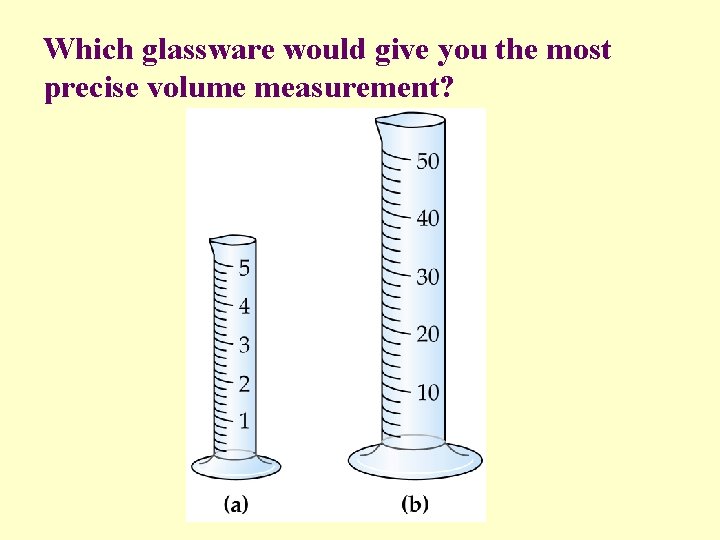
Which glassware would give you the most precise volume measurement?

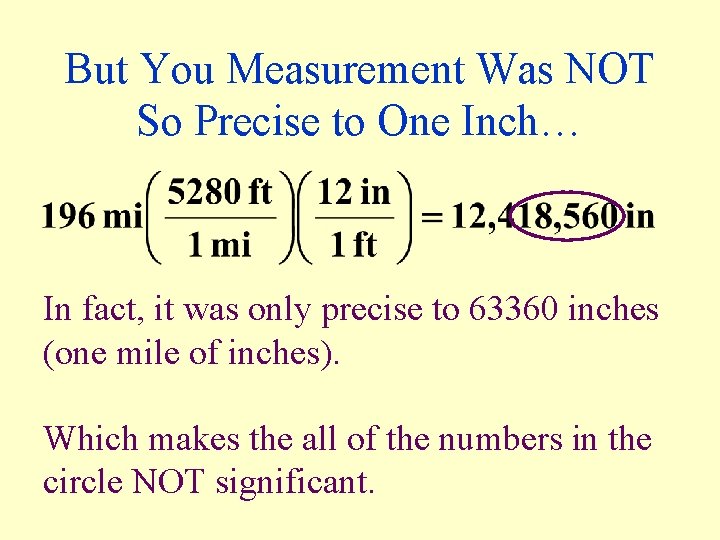
Never Use Too Many Figures… • If you express a the distance from Austin to Dallas as 196 miles 1 mile (the last digit is estimated). • If you choose to report this measurement in inches, your calculator would show:

But You Measurement Was NOT So Precise to One Inch… In fact, it was only precise to 63360 inches (one mile of inches). Which makes the all of the numbers in the circle NOT significant.

In ALL LABS… • Report all certain values as significant figures. • Estimate (and report as significant) to the 1/10 th of the smallest division of measurement.

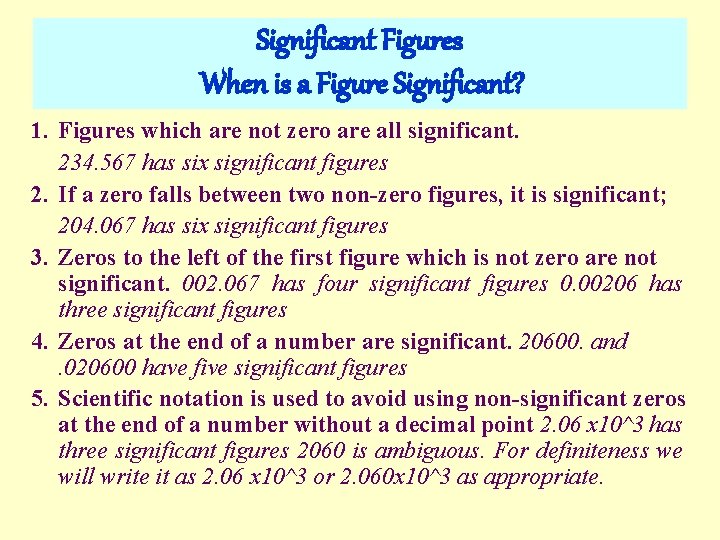
Significant Figures When is a Figure Significant? 1. Figures which are not zero are all significant. 234. 567 has six significant figures 2. If a zero falls between two non-zero figures, it is significant; 204. 067 has six significant figures 3. Zeros to the left of the first figure which is not zero are not significant. 002. 067 has four significant figures 0. 00206 has three significant figures 4. Zeros at the end of a number are significant. 20600. and. 020600 have five significant figures 5. Scientific notation is used to avoid using non-significant zeros at the end of a number without a decimal point 2. 06 x 10^3 has three significant figures 2060 is ambiguous. For definiteness we will write it as 2. 06 x 10^3 or 2. 060 x 10^3 as appropriate.

Significant Figures Rules of Operation ADDING AND SUBTRACTING When measurements are added or subtracted, the answer can contain no more decimal places than the least precise measurement. 150. 0 g H 2 O + 0. 507 g salt 150. 5 g solution

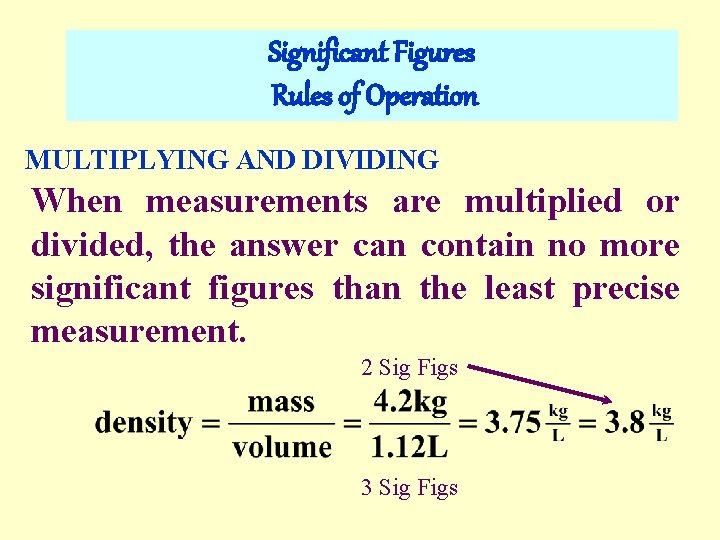
Significant Figures Rules of Operation MULTIPLYING AND DIVIDING When measurements are multiplied or divided, the answer can contain no more significant figures than the least precise measurement. 2 Sig Figs 3 Sig Figs
 There's no such thing as a free lunch artinya
There's no such thing as a free lunch artinya There is no such thing as absolute security
There is no such thing as absolute security There is no such thing as a bad kid
There is no such thing as a bad kid I ask the lord
I ask the lord There is no wrong way to do the right thing
There is no wrong way to do the right thing Speaking mathematically variables
Speaking mathematically variables There was a man from russia long ago
There was a man from russia long ago Existential universal statements examples
Existential universal statements examples There are some cake
There are some cake Ecological succession
Ecological succession Cheese contable o incontable
Cheese contable o incontable What part of speech is open
What part of speech is open There is there are
There is there are There is there are
There is there are Pep unit
Pep unit There is there are negative form
There is there are negative form There
There Demonstrativos
Demonstrativos