Measurement Notes Measurement Notes Accuracy As close to






- Slides: 17

Measurement Notes

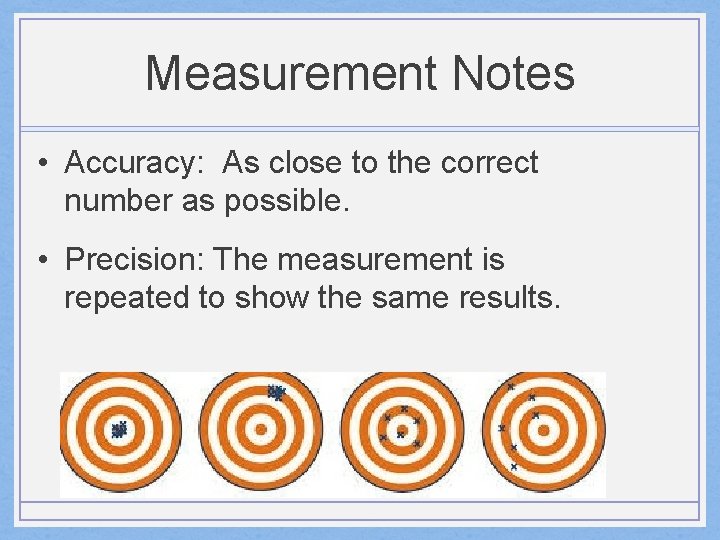
Measurement Notes • Accuracy: As close to the correct number as possible. • Precision: The measurement is repeated to show the same results.

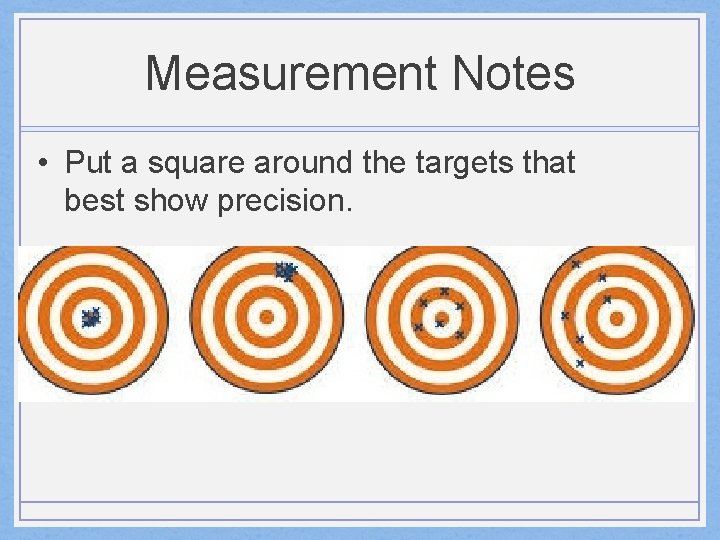
Measurement Notes • Put a square around the targets that best show precision.

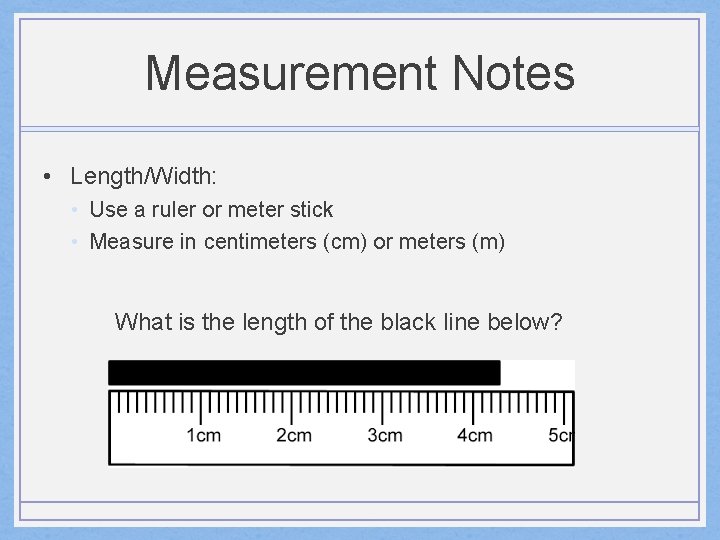
Measurement Notes • Length/Width: • Use a ruler or meter stick • Measure in centimeters (cm) or meters (m) What is the length of the black line below?

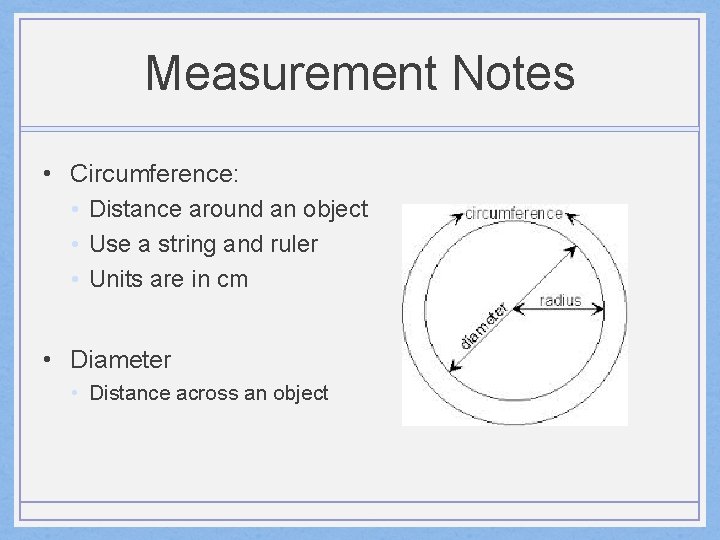
Measurement Notes • Circumference: • Distance around an object • Use a string and ruler • Units are in cm • Diameter • Distance across an object

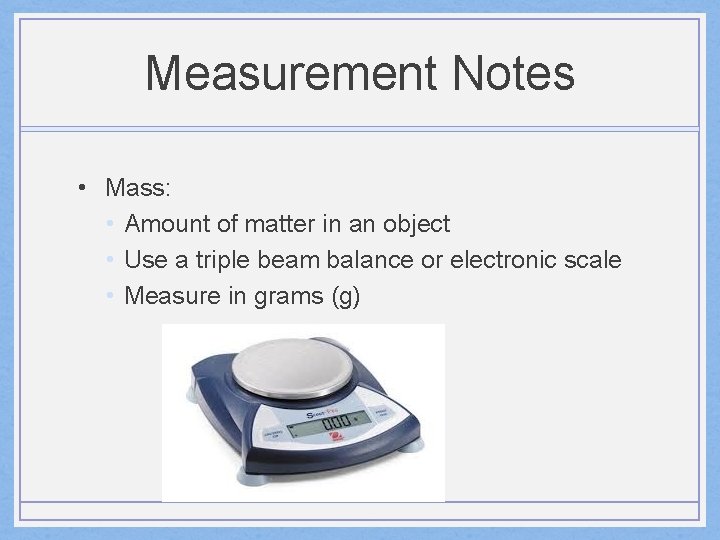
Measurement Notes • Mass: • Amount of matter in an object • Use a triple beam balance or electronic scale • Measure in grams (g)

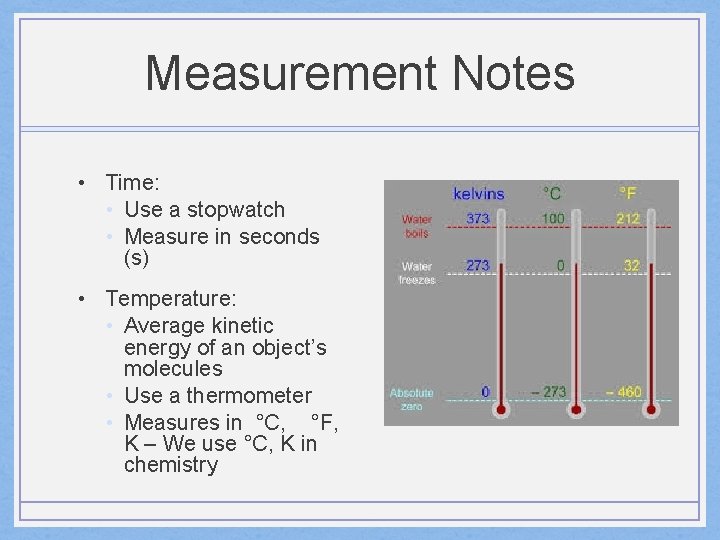
Measurement Notes • Time: • Use a stopwatch • Measure in seconds (s) • Temperature: • Average kinetic energy of an object’s molecules • Use a thermometer • Measures in °C, °F, K – We use °C, K in chemistry

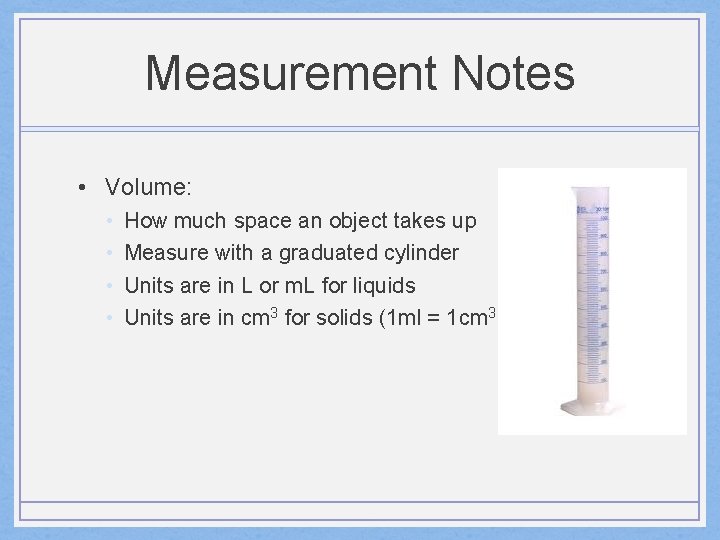
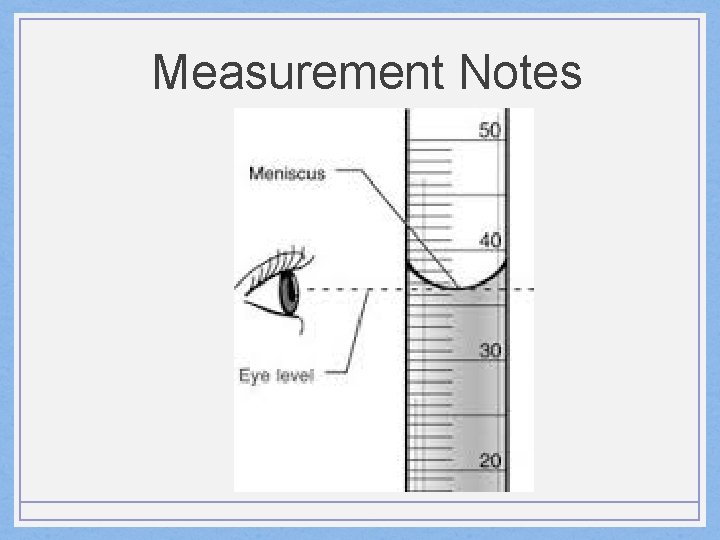
Measurement Notes • Volume: • • How much space an object takes up Measure with a graduated cylinder Units are in L or m. L for liquids Units are in cm 3 for solids (1 ml = 1 cm 3 )

Measurement Notes

Measurement Notes

Measurement Notes

Measurement Notes • Volume by Displacement: • Used for objects with irregular shapes • 1. ) Fill a graduated cylinder with 50 m. L of water • 2. ) Drop object carefully into water • 3. ) Record new volume in graduated cylinder • 4. ) Subtract initial volume from the final volume of 50 m. L to get the volume of the object Volume of object = VF - VI

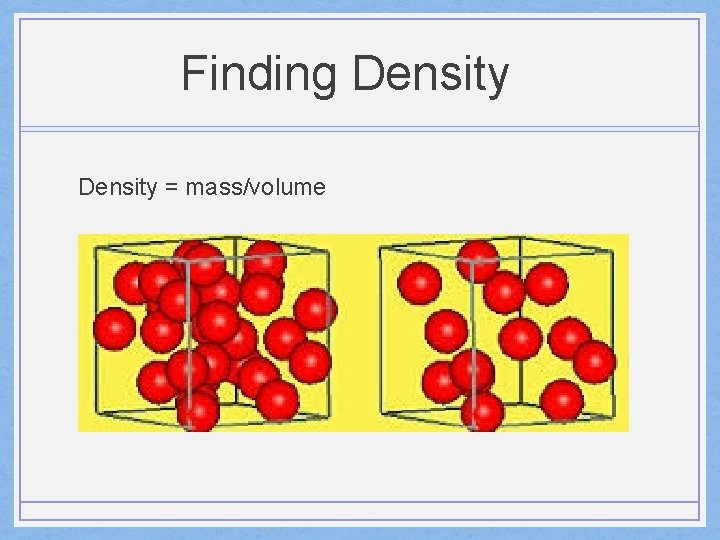
Finding Density = mass/volume

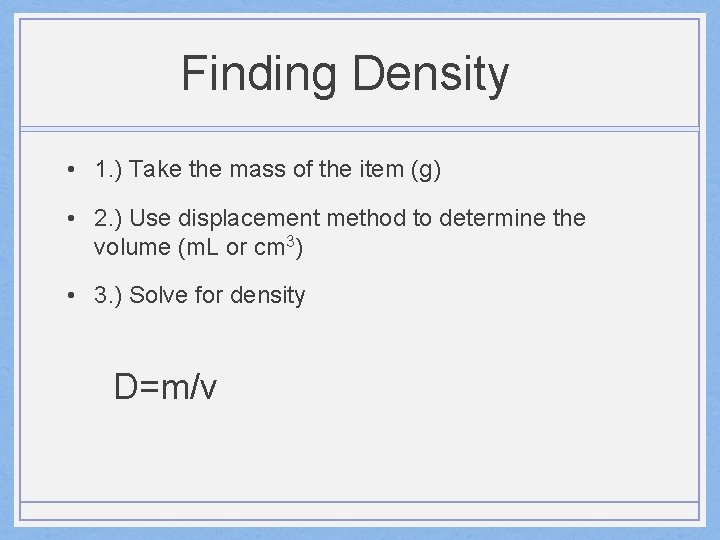
Finding Density • 1. ) Take the mass of the item (g) • 2. ) Use displacement method to determine the volume (m. L or cm 3) • 3. ) Solve for density D=m/v

Density • A ball has a mass of 20. 1 g and a volume of 5. 2 cm 3. What is its density?

Density The density of water = 1. 0 g/m. L • If the density of an object > 1. 0 g/cm 3 what will happen? • If the density of an object < 1. 0 g/cm 3 what will happen?

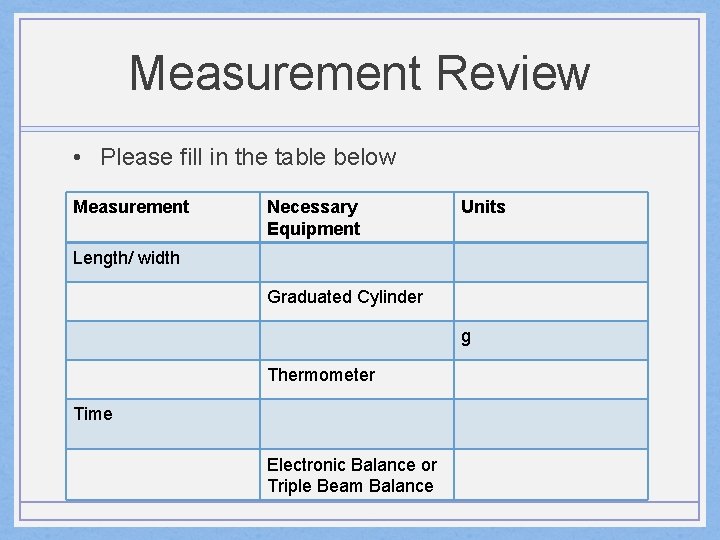
Measurement Review • Please fill in the table below Measurement Necessary Equipment Units Length/ width Graduated Cylinder g Thermometer Time Electronic Balance or Triple Beam Balance