Measurement measurement quantity with number and a unit

Measurement measurement: quantity with number _______ and a unit _____. 42. 5 g 1. 05 m. L 16 cm

Scientific Notation In scientific notation all numbers are written in the form m × 10 n (m times ten raised to the power of n), where the exponent n is an integer, and the coefficient m is any real number between 1 inclusive and 10. that is 1 and 9. 999999999… Another example: 431000 m = 4. 31 x 105 m a really big number: 100, 000, 000 (atoms in a cell) or really small number: 0. 000 000 01 meter (size of one atom) can be written without (a lot of) zeroes by using powers of 10.
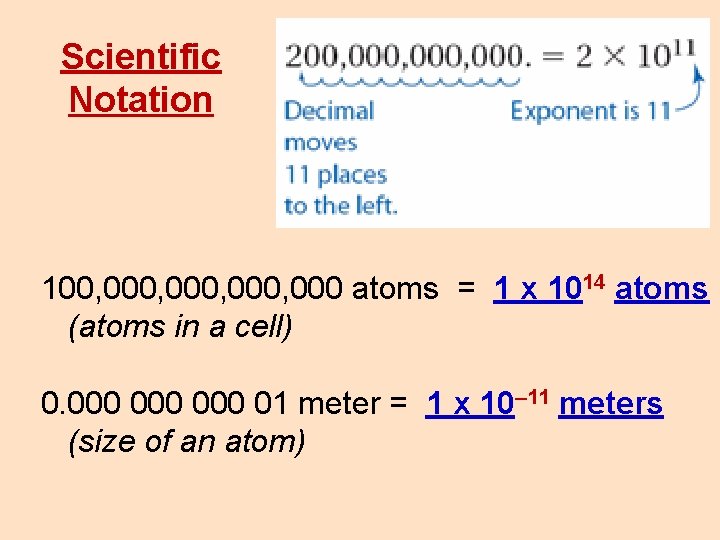
Scientific Notation 100, 000, 000 atoms = 1 x 1014 atoms (atoms in a cell) 0. 000 000 01 meter = 1 x 10– 11 meters (size of an atom)
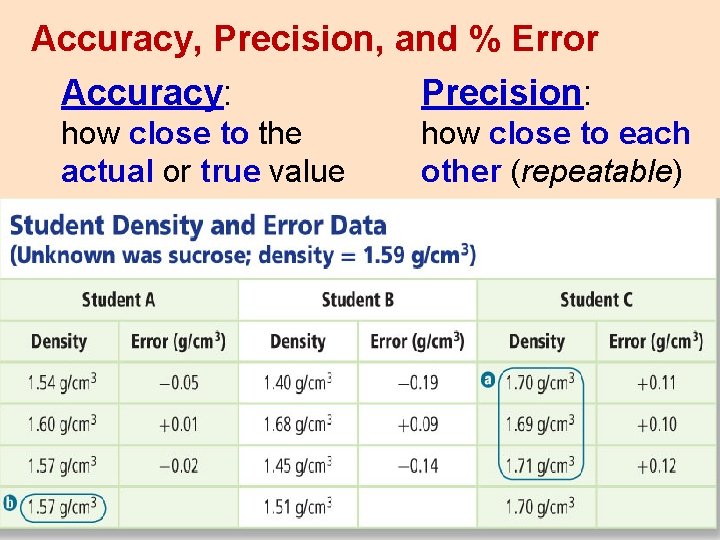
Accuracy, Precision, and % Error Accuracy: Precision: how close to the actual or true value how close to each other (repeatable)

Accurate and _______ Precise but not ____ Accurate Neither Precise Nor Accurate
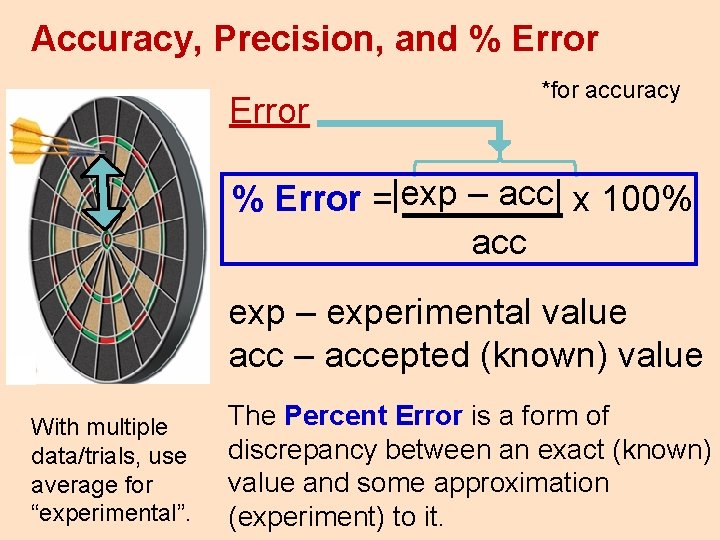
Accuracy, Precision, and % Error *for accuracy – acc| x 100% % Error =|exp ____ acc exp – experimental value acc – accepted (known) value With multiple data/trials, use average for “experimental”. The Percent Error is a form of discrepancy between an exact (known) value and some approximation (experiment) to it.
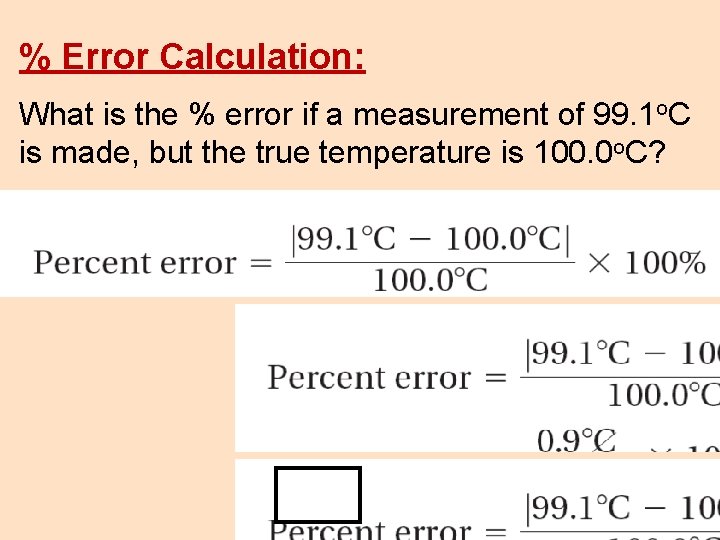
% Error Calculation: What is the % error if a measurement of 99. 1 o. C is made, but the true temperature is 100. 0 o. C?

Quick Quiz! 1. Which of the following expressions has NOT been correctly changed to sci. not. ? A. 0. 00456 = 4. 56 10– 3 B. 0. 0000254 = 2. 54 10– 5 C. 8, 426, 000 = 8. 426 106 D. 45, 200 = 4. 52 103
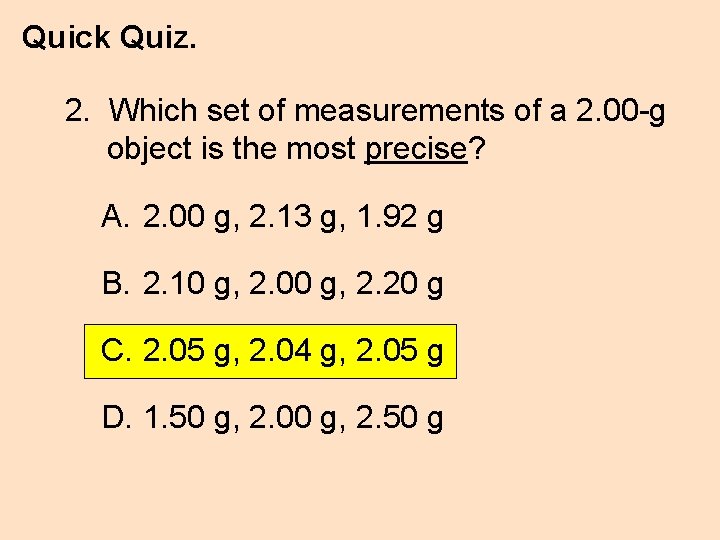
Quick Quiz. 2. Which set of measurements of a 2. 00 -g object is the most precise? A. 2. 00 g, 2. 13 g, 1. 92 g B. 2. 10 g, 2. 00 g, 2. 20 g C. 2. 05 g, 2. 04 g, 2. 05 g D. 1. 50 g, 2. 00 g, 2. 50 g
- Slides: 9