Measurement In Science Certainty in Measurement Accuracy Precision





- Slides: 21

Measurement In Science: Certainty in Measurement: Accuracy, Precision and Significant Figures Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Certainty in measurement When measuring quantitative properties of matter you must use a measuring instrument. Measuring instruments are generally graduated- made with marks to show levels of amounts Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All right reserved.

Certainty in measurement Some measuring instruments are more exact than others. This means that every instrument has a certain level of uncertainty- inexactness It is important to be clear about how exact and reliable a measurement is, so that you can decide whether it is trustworthy or not Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

ACCURACY vs. PRECISION • Accuracy indicates how close a measurement is to the real amount. • Precision indicates how close together or how repeatable the results are. • A precise measuring instrument will give very nearly the same result each time it is used. ( Of course the repeated measurement, may or may not be accurate!)

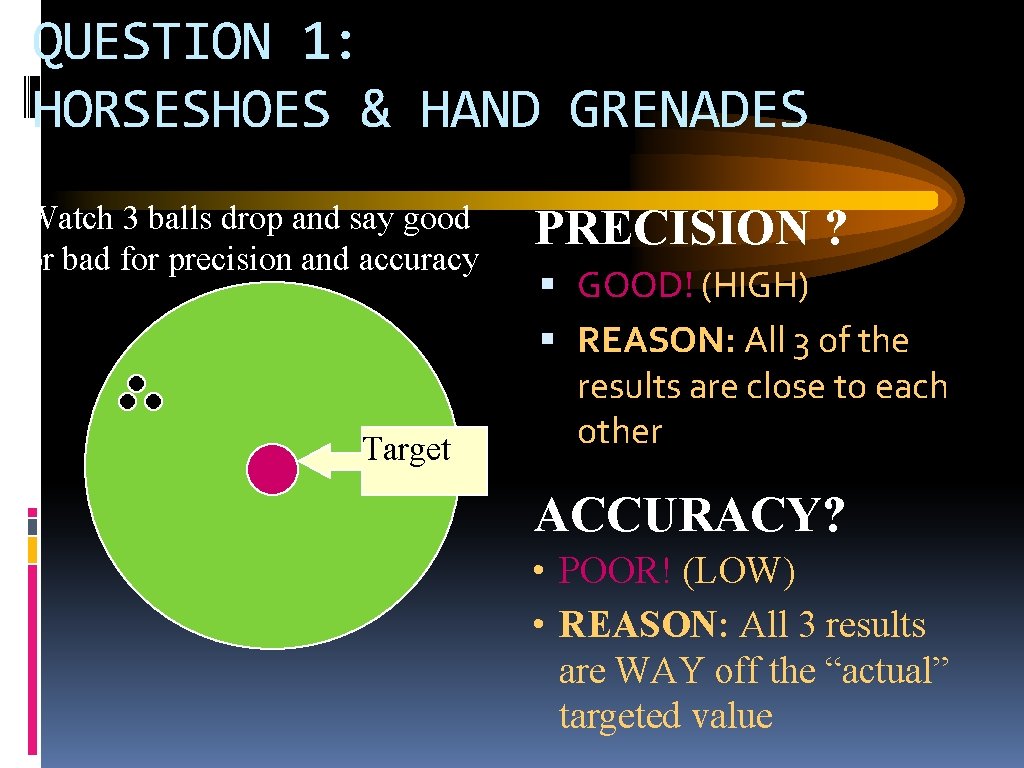
QUESTION 1: HORSESHOES & HAND GRENADES Watch 3 balls drop and say good or bad for precision and accuracy Target PRECISION ? GOOD! (HIGH) REASON: All 3 of the results are close to each other ACCURACY? • POOR! (LOW) • REASON: All 3 results are WAY off the “actual” targeted value

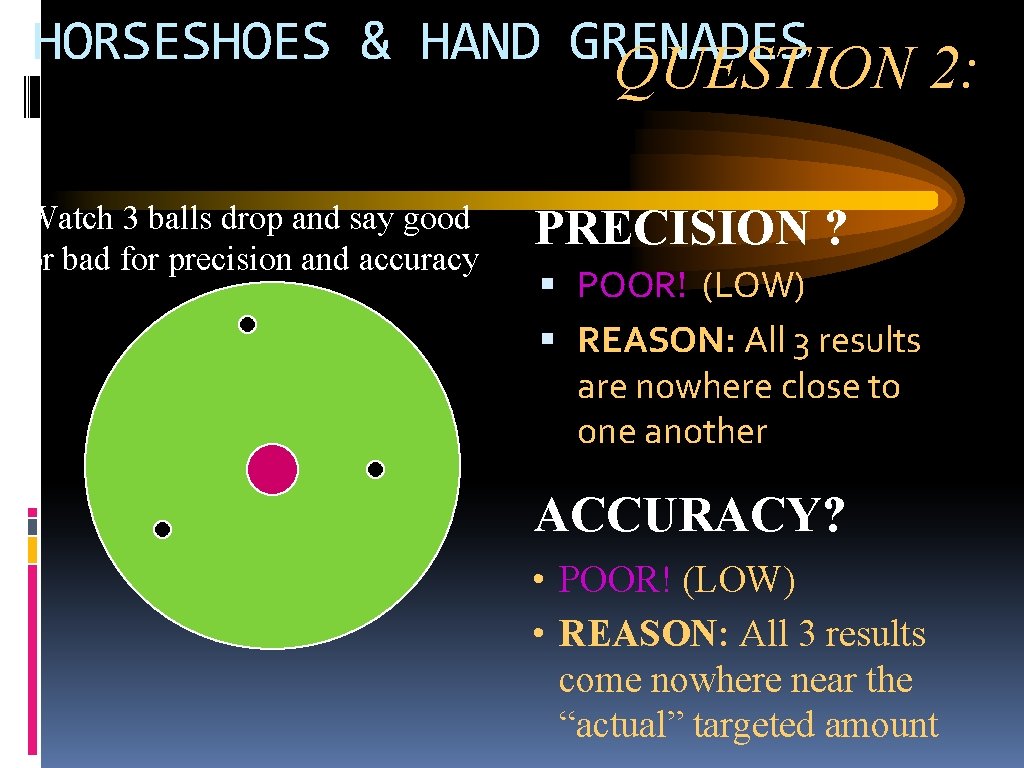
HORSESHOES & HAND GRENADES QUESTION 2: Watch 3 balls drop and say good or bad for precision and accuracy PRECISION ? POOR! (LOW) REASON: All 3 results are nowhere close to one another ACCURACY? • POOR! (LOW) • REASON: All 3 results come nowhere near the “actual” targeted amount

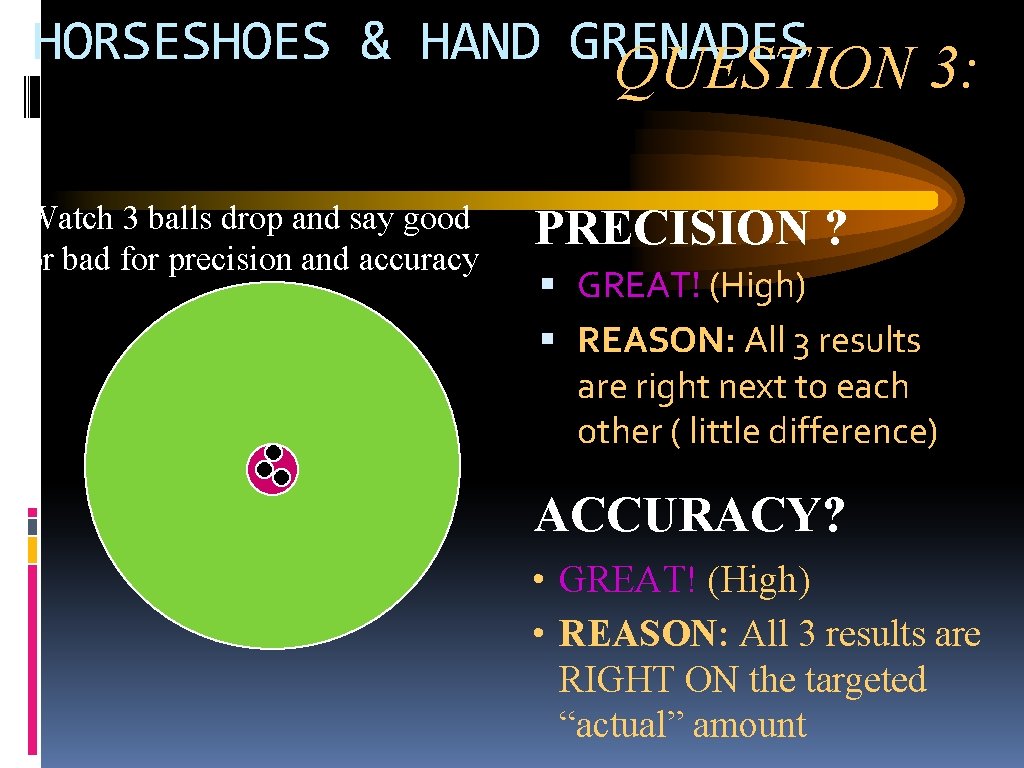
HORSESHOES & HAND GRENADES QUESTION 3: Watch 3 balls drop and say good or bad for precision and accuracy PRECISION ? GREAT! (High) REASON: All 3 results are right next to each other ( little difference) ACCURACY? • GREAT! (High) • REASON: All 3 results are RIGHT ON the targeted “actual” amount

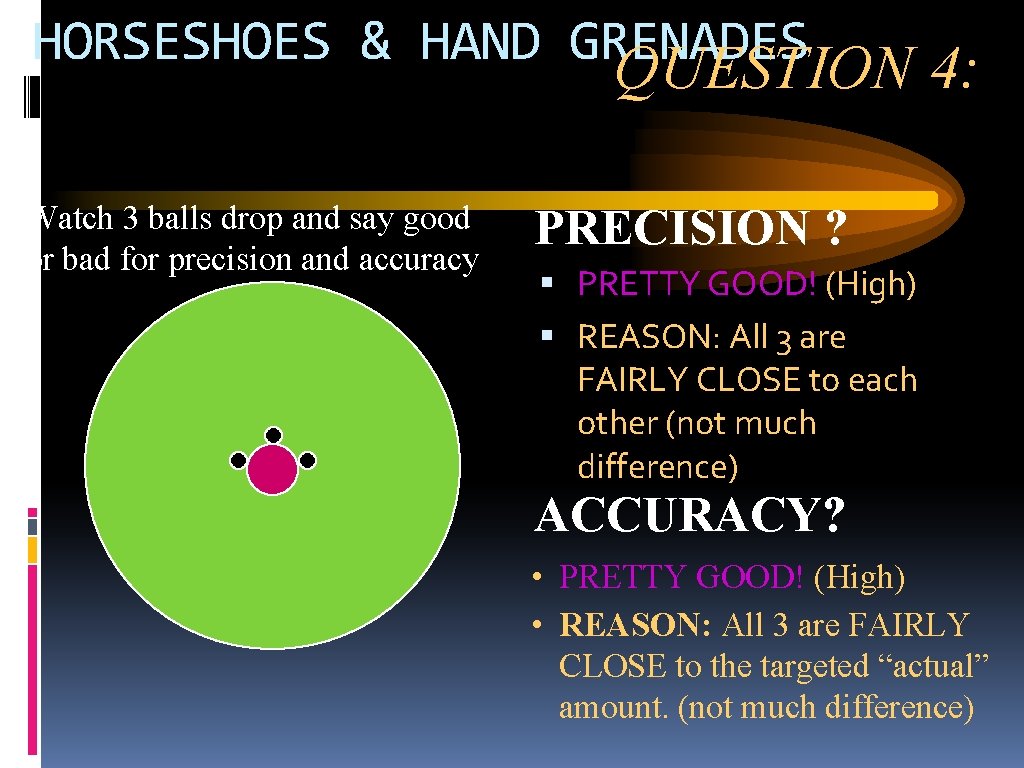
HORSESHOES & HAND GRENADES QUESTION 4: Watch 3 balls drop and say good or bad for precision and accuracy PRECISION ? PRETTY GOOD! (High) REASON: All 3 are FAIRLY CLOSE to each other (not much difference) ACCURACY? • PRETTY GOOD! (High) • REASON: All 3 are FAIRLY CLOSE to the targeted “actual” amount. (not much difference)

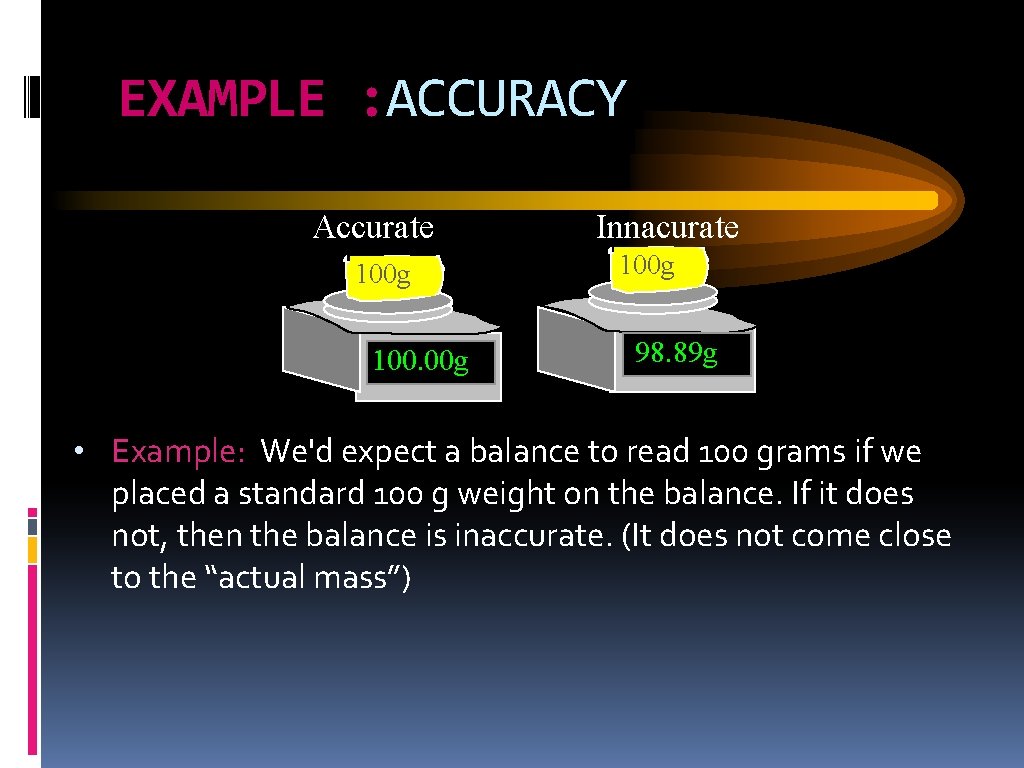
EXAMPLE : ACCURACY Accurate 100 g 100. 00 g Innacurate 100 g 98. 89 g • Example: We'd expect a balance to read 100 grams if we placed a standard 100 g weight on the balance. If it does not, then the balance is inaccurate. (It does not come close to the “actual mass”)

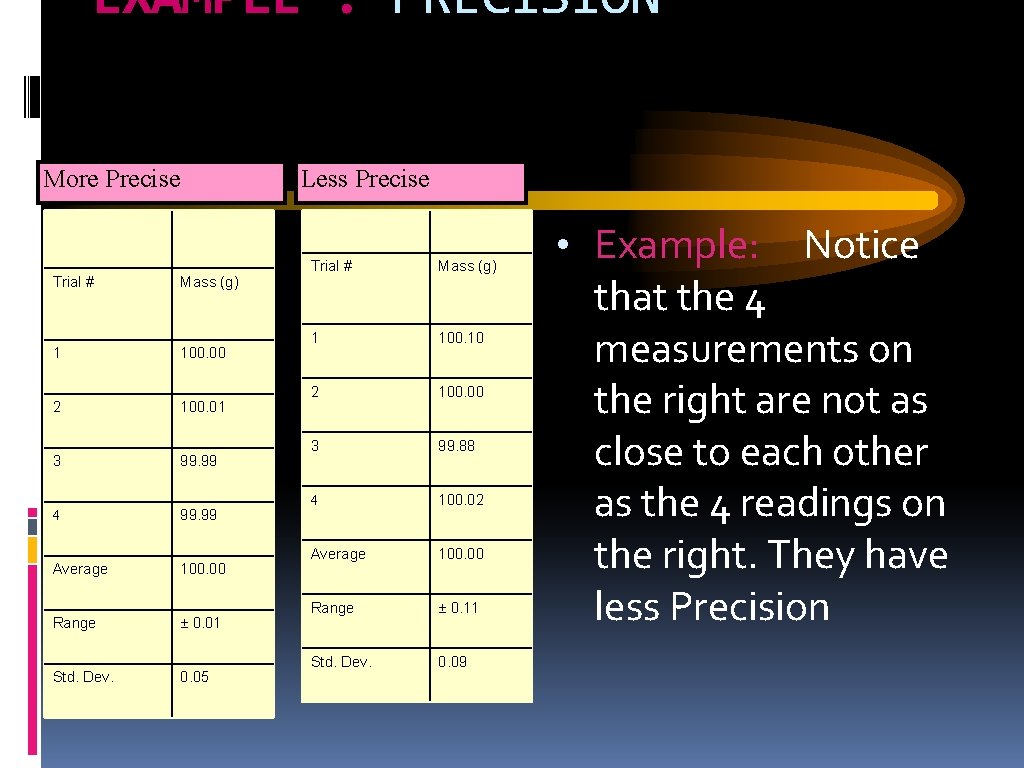
EXAMPLE : PRECISION More Precise Trial # 1 2 3 4 Average Range Std. Dev. Mass (g) 100. 00 100. 01 99. 99 100. 00 ± 0. 01 0. 05 Less Precise Trial # Mass (g) 1 100. 10 2 100. 00 3 99. 88 4 100. 02 Average 100. 00 Range ± 0. 11 Std. Dev. 0. 09 • Example: Notice that the 4 measurements on the right are not as close to each other as the 4 readings on the right. They have less Precision

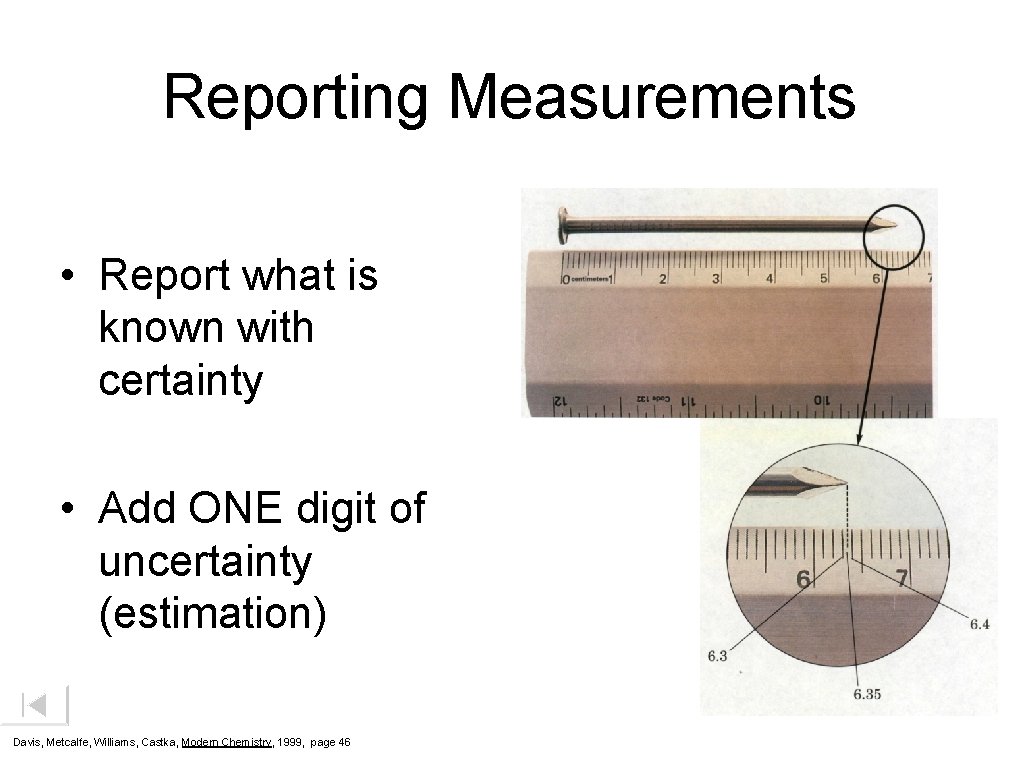
Reporting Measurements • Report what is known with certainty • Add ONE digit of uncertainty (estimation) Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 46

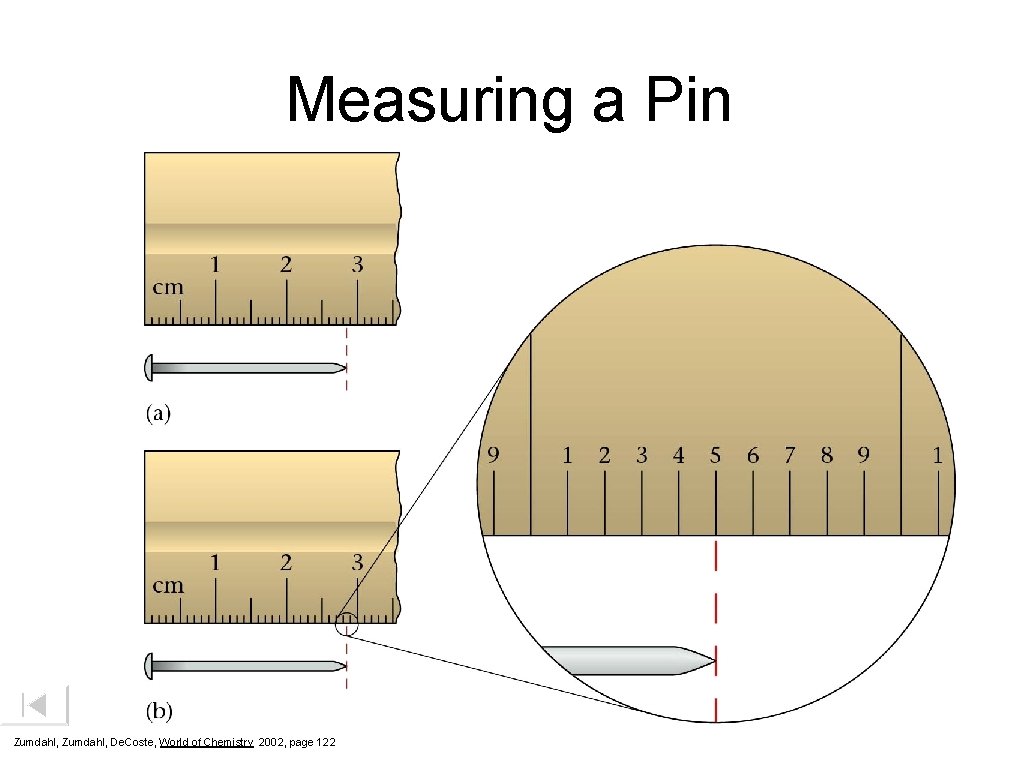
Measuring a Pin Zumdahl, De. Coste, World of Chemistry 2002, page 122

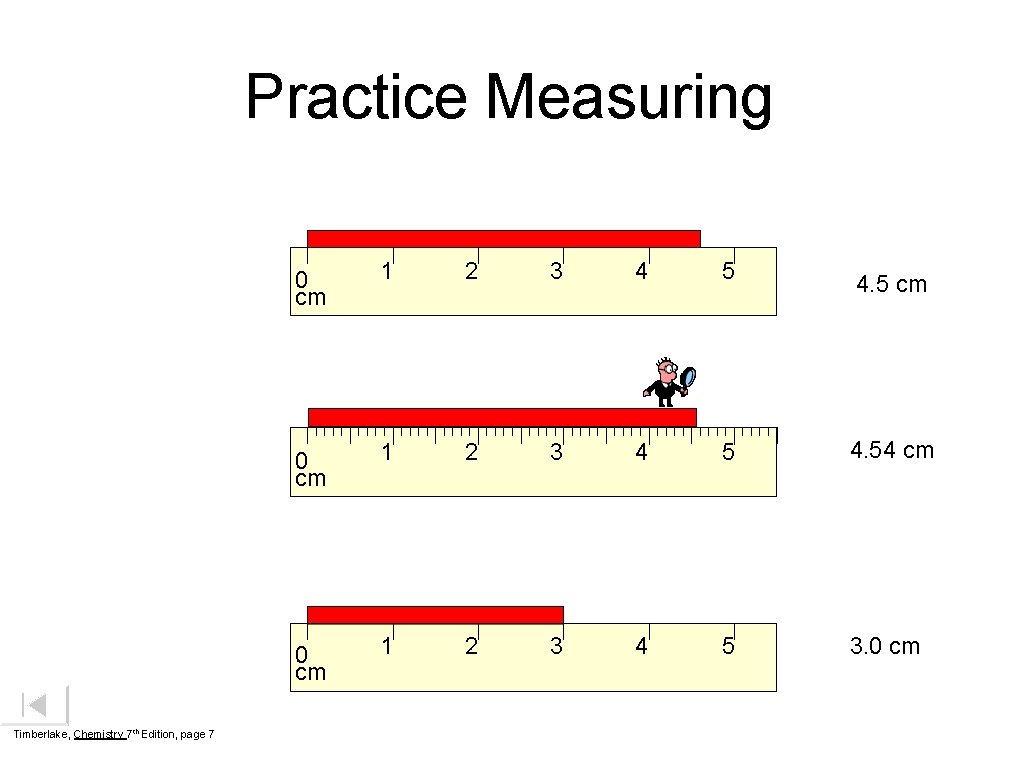
Practice Measuring Timberlake, Chemistry 7 th Edition, page 7 0 cm 1 2 3 4 5 4. 54 cm 0 cm 1 2 3 4 5 3. 0 cm 4. 5 cm

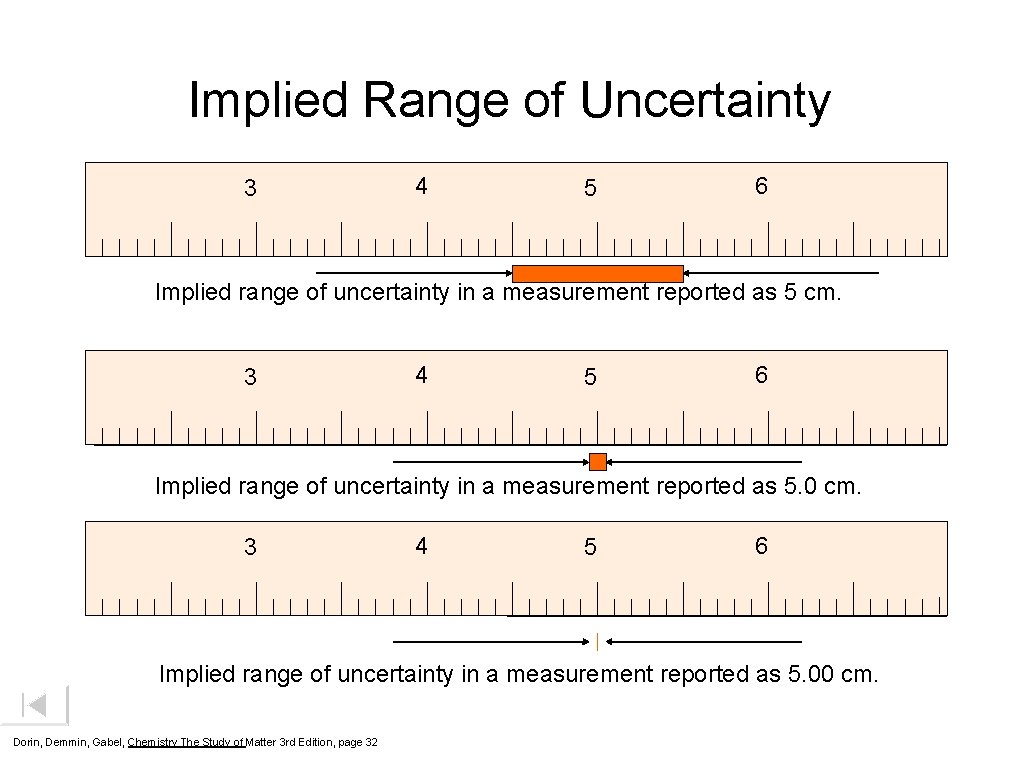
Implied Range of Uncertainty 3 4 5 6 Implied range of uncertainty in a measurement reported as 5 cm. 3 4 5 6 Implied range of uncertainty in a measurement reported as 5. 00 cm. Dorin, Demmin, Gabel, Chemistry The Study of Matter 3 rd Edition, page 32

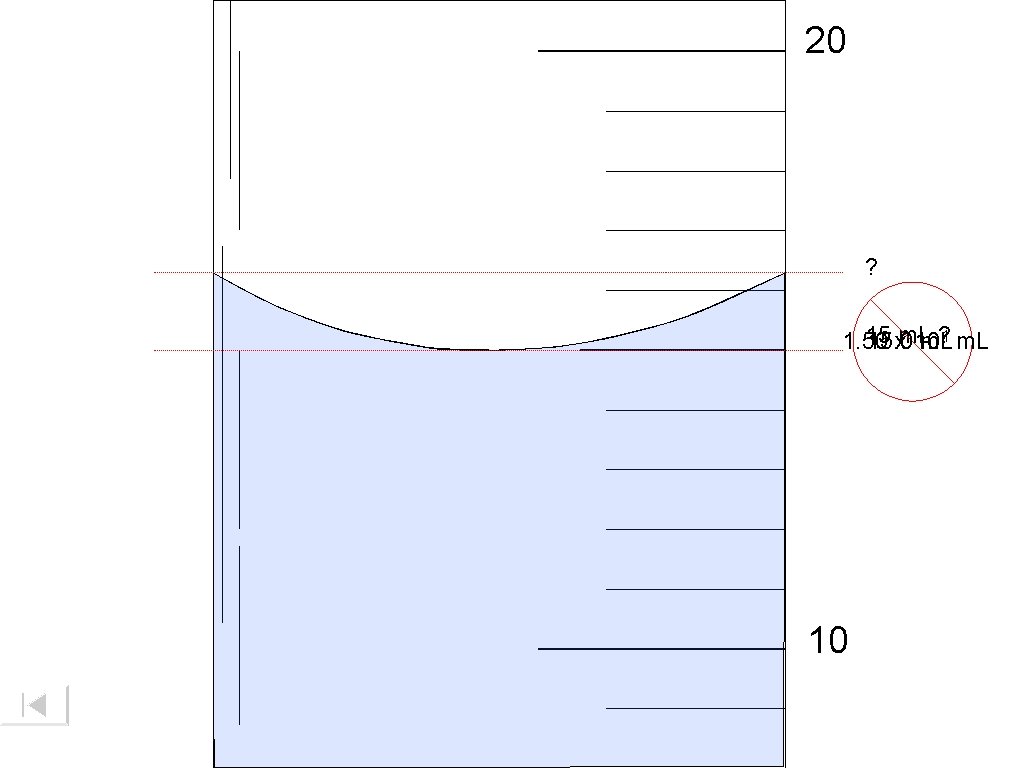
20 ? 15 ? 1 m. L 1. 50 15. 0 xm. L 10

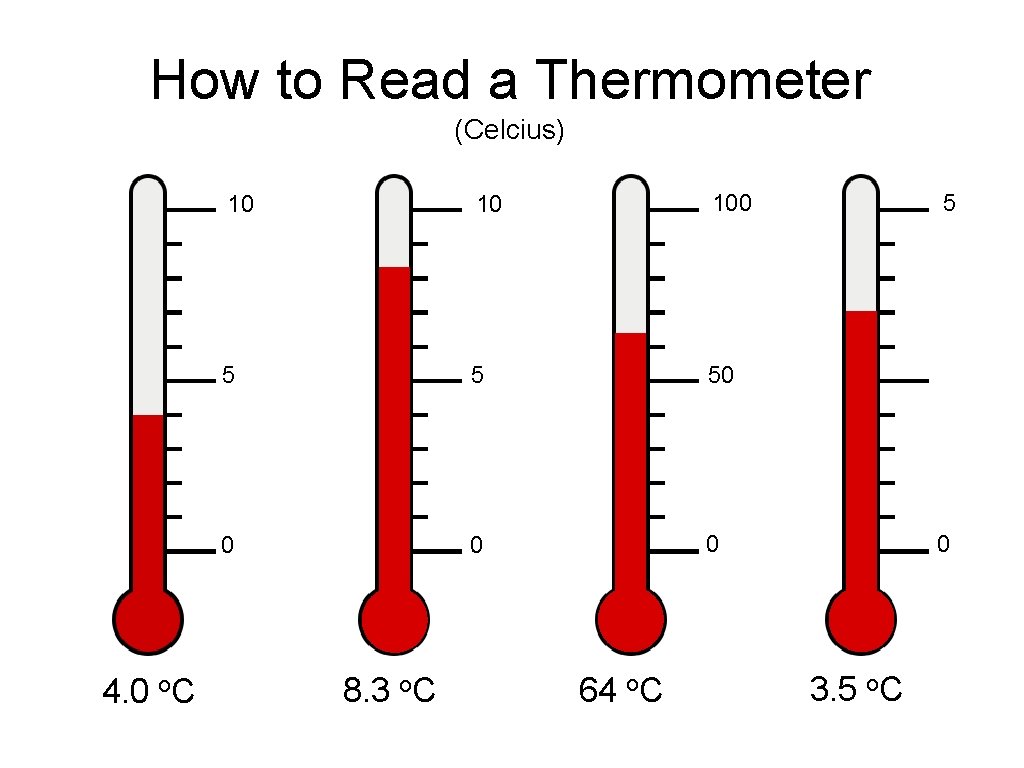
How to Read a Thermometer (Celcius) 4. 0 o. C 10 10 100 5 5 50 0 8. 3 o. C 64 o. C 5 0 3. 5 o. C

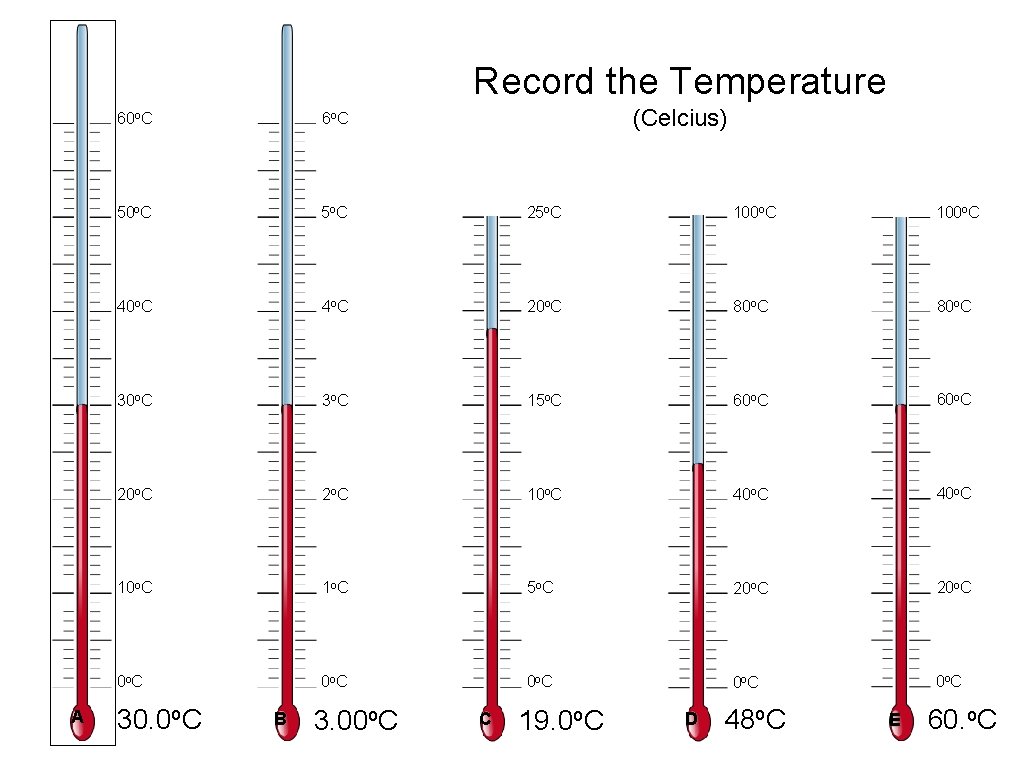
Record the Temperature A (Celcius) 60 o. C 6 o C 50 o. C 5 o C 25 o. C 100 o. C 40 o. C 4 o C 20 o. C 80 o. C 3 o C 15 o. C 60 o. C 20 o. C 2 o C 10 o. C 40 o. C 1 o C 5 o C 20 o. C 0 o C 0 o C 30. 0 o. C B 3. 00 o. C C 19. 0 o. C D 48 o. C E 60. o. C

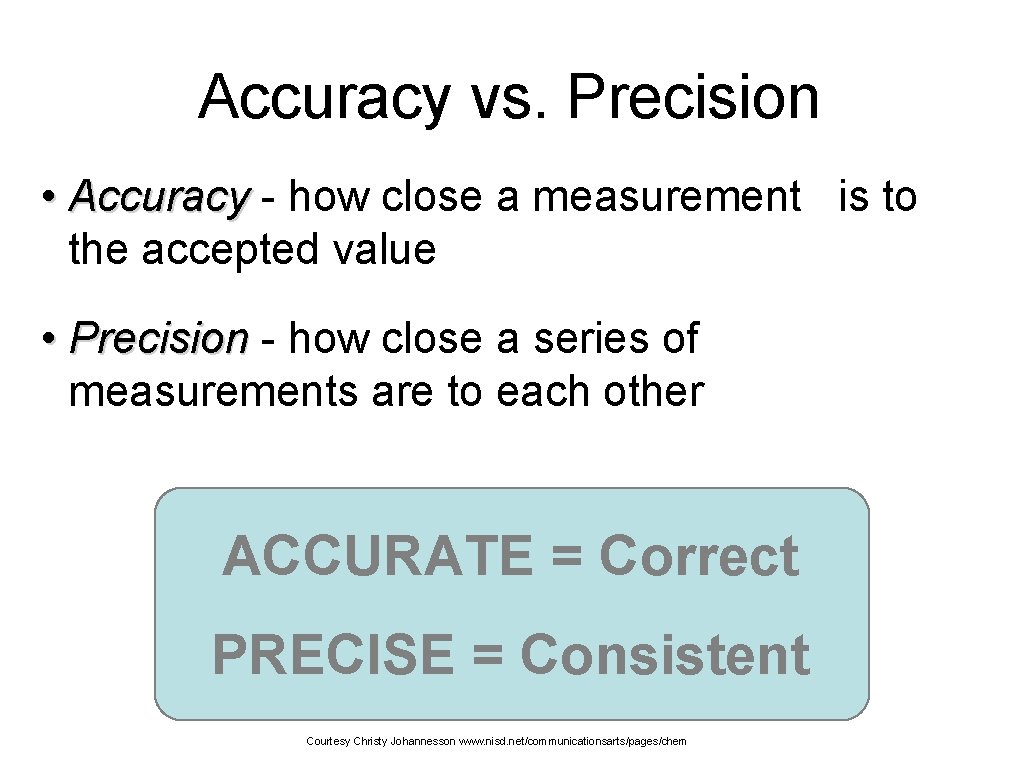
Accuracy vs. Precision • Accuracy - how close a measurement is to the accepted value • Precision - how close a series of measurements are to each other ACCURATE = Correct PRECISE = Consistent Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

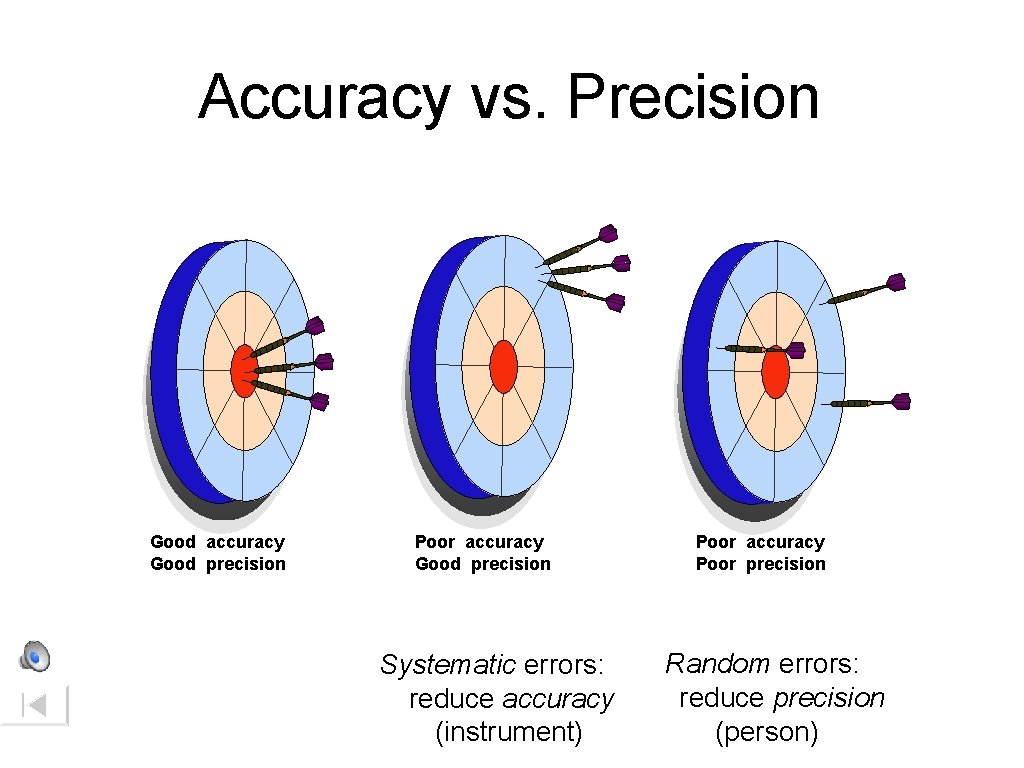
Accuracy vs. Precision Good accuracy Good precision Poor accuracy Good precision Systematic errors: reduce accuracy (instrument) Poor accuracy Poor precision Random errors: reduce precision (person)

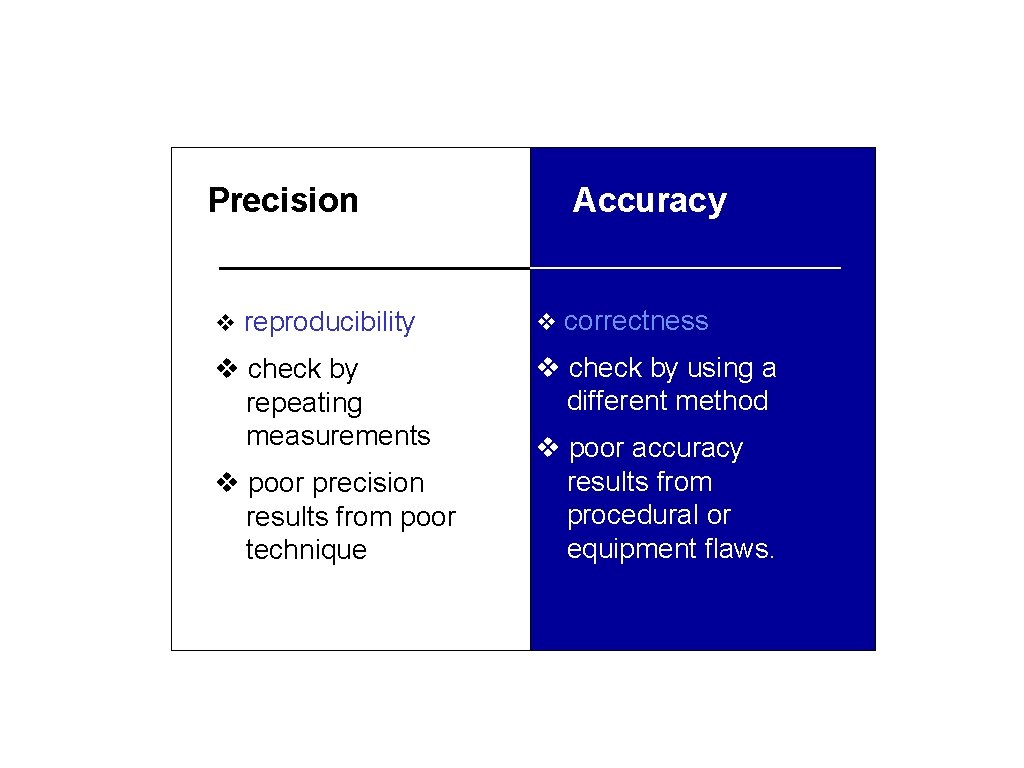
Precision Accuracy v reproducibility v correctness v check by repeating measurements v check by using a different method v poor precision results from poor technique v poor accuracy results from procedural or equipment flaws.

Types of errors Systematic • Instrument not ‘zeroed’ properly • Reagents made at wrong concentration Random • Temperature in room varies ‘wildly’ • Person running test is not properly trained