MEASUREMENT I Using Measurements A Accuracy and Precision




- Slides: 16

MEASUREMENT I. Using Measurements A. Accuracy and Precision B. Percent Error I C. Significant Figures II D. Scientific Notation III E. Proportions C. Johannesson

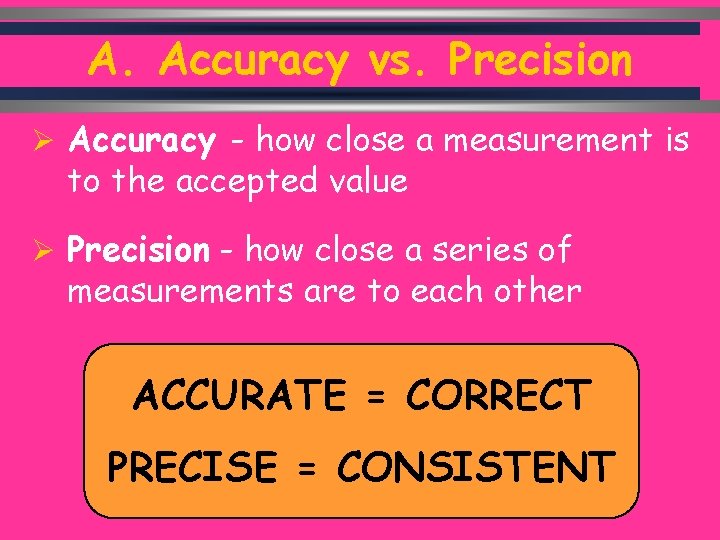
A. Accuracy vs. Precision Ø Accuracy - how close a measurement is to the accepted value Ø Precision - how close a series of measurements are to each other ACCURATE = CORRECT PRECISE = CONSISTENT C. Johannesson

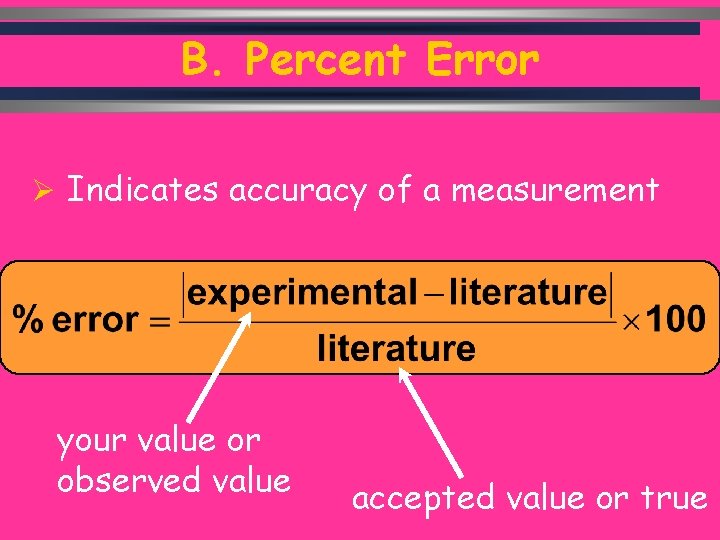
B. Percent Error Ø Indicates accuracy of a measurement your value or observed value accepted value or true C. Johannesson

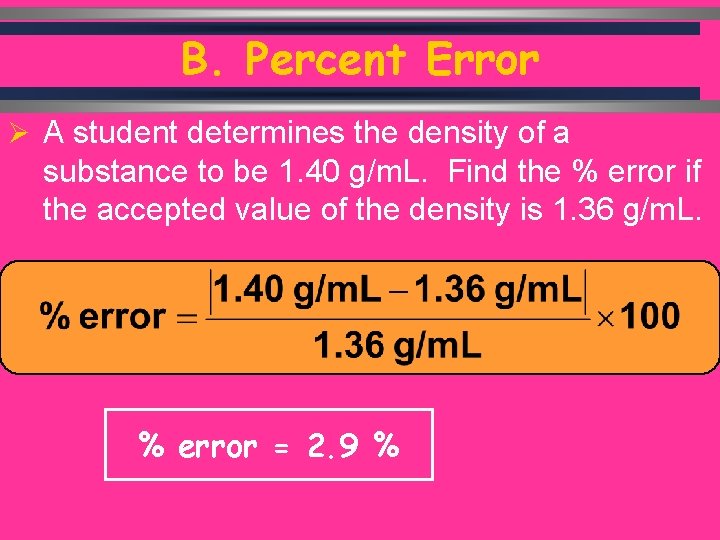
B. Percent Error Ø A student determines the density of a substance to be 1. 40 g/m. L. Find the % error if the accepted value of the density is 1. 36 g/m. L. % error = 2. 9 %

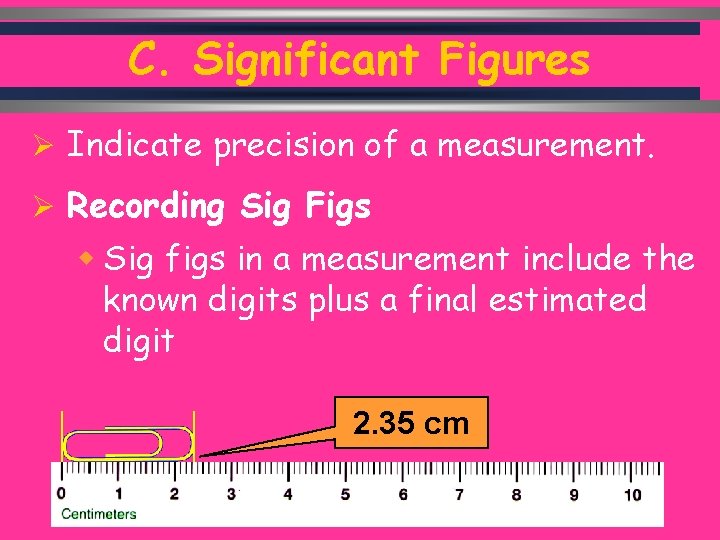
C. Significant Figures Ø Indicate precision of a measurement. Ø Recording Sig Figs w Sig figs in a measurement include the known digits plus a final estimated digit 2. 35 cm C. Johannesson

C. Significant Figures Ø Counting Sig Figs (See page 47) w Count all numbers EXCEPT: ² Leading zeros -- 0. 0025 ² Trailing zeros without a decimal point -- 2, 500 C. Johannesson

C. Significant Figures Counting Sig Fig Examples 1. 23. 50 4 sig figs 2. 402 3 sig figs 3. 5, 280 3 sig figs 4. 0. 080 2 sig figs C. Johannesson

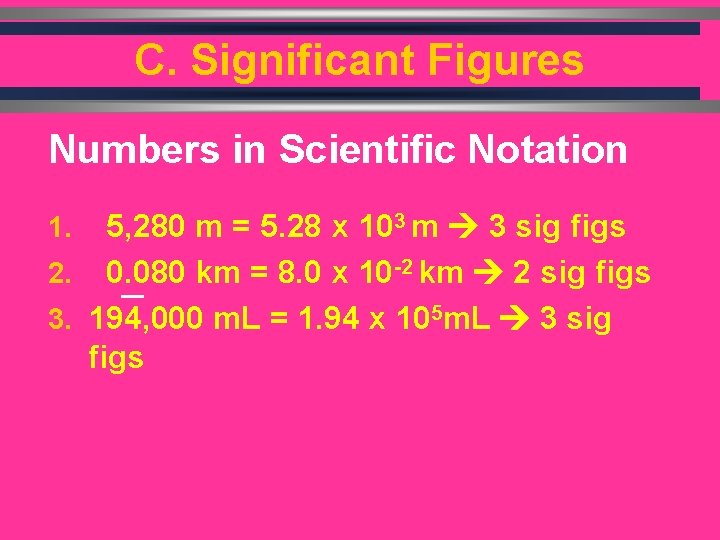
C. Significant Figures Numbers in Scientific Notation 5, 280 m = 5. 28 x 103 m 3 sig figs 2. 0. 080 km = 8. 0 x 10 -2 km 2 sig figs 3. 194, 000 m. L = 1. 94 x 105 m. L 3 sig figs 1.

C. Significant Figures Ø Calculating with Sig Figs w Multiply/Divide - The # with the fewest sig figs determines the # of sig figs in the answer. (13. 91 g/cm 3)(23. 3 cm 3) = 324. 103 g 4 SF 324 g C. Johannesson

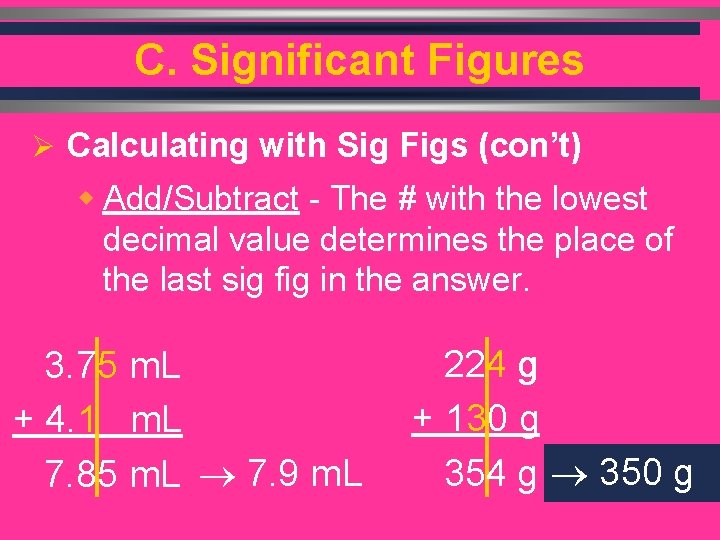
C. Significant Figures Ø Calculating with Sig Figs (con’t) w Add/Subtract - The # with the lowest decimal value determines the place of the last sig fig in the answer. 3. 75 m. L + 4. 1 m. L 7. 85 m. L 7. 9 m. L C. Johannesson 224 g + 130 g 354 g 350 g

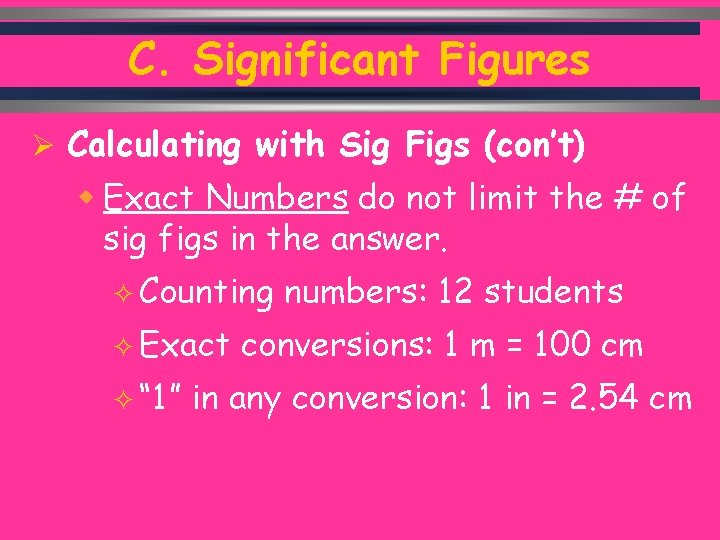
C. Significant Figures Ø Calculating with Sig Figs (con’t) w Exact Numbers do not limit the # of sig figs in the answer. ² Counting ² Exact ² “ 1” numbers: 12 students conversions: 1 m = 100 cm in any conversion: 1 in = 2. 54 cm C. Johannesson

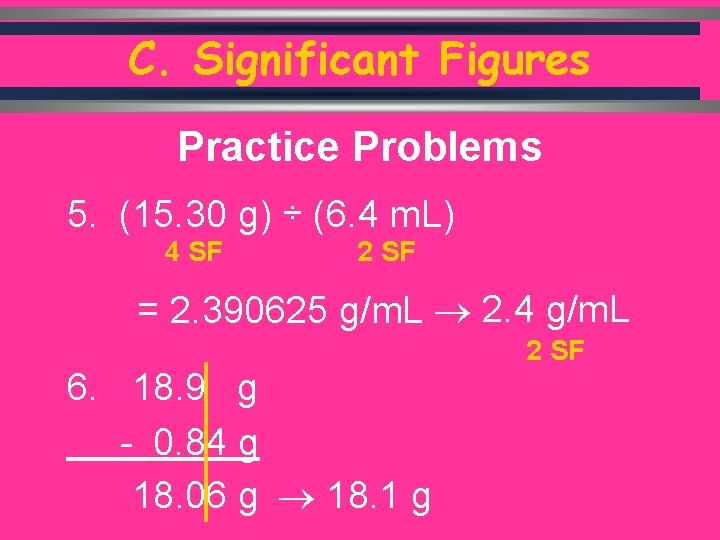
C. Significant Figures Practice Problems 5. (15. 30 g) ÷ (6. 4 m. L) 4 SF 2 SF = 2. 390625 g/m. L 2. 4 g/m. L 2 SF 6. 18. 9 g - 0. 84 g 18. 06 g 18. 1 g C. Johannesson

D. Scientific Notation 65, 000 kg 6. 5 × 104 kg Ø Converting into Sci. Notation: w Move decimal until there’s 1 digit to its left. Places moved = exponent. w Large # (>1) positive exponent Small # (<1) negative exponent C. Johannesson w Only include sig figs.

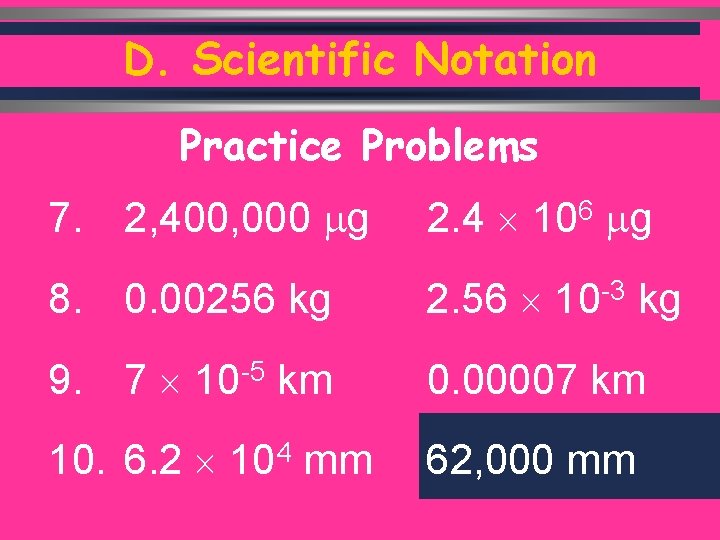
D. Scientific Notation Practice Problems 7. 2, 400, 000 g 2. 4 8. 0. 00256 kg 2. 56 9. 7 10 -5 km 0. 00007 km 10. 6. 2 104 mm 62, 000 mm C. Johannesson 6 10 g -3 10 kg

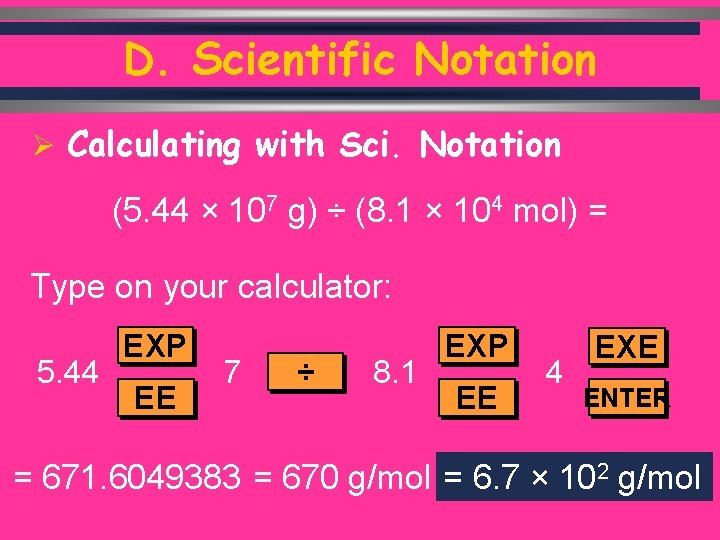
D. Scientific Notation Ø Calculating with Sci. Notation (5. 44 × 107 g) ÷ (8. 1 × 104 mol) = Type on your calculator: 5. 44 EXP EE 7 ÷ 8. 1 EXP EE 4 EXE ENTER = 671. 6049383 = 670 g/mol = 6. 7 × 102 g/mol C. Johannesson

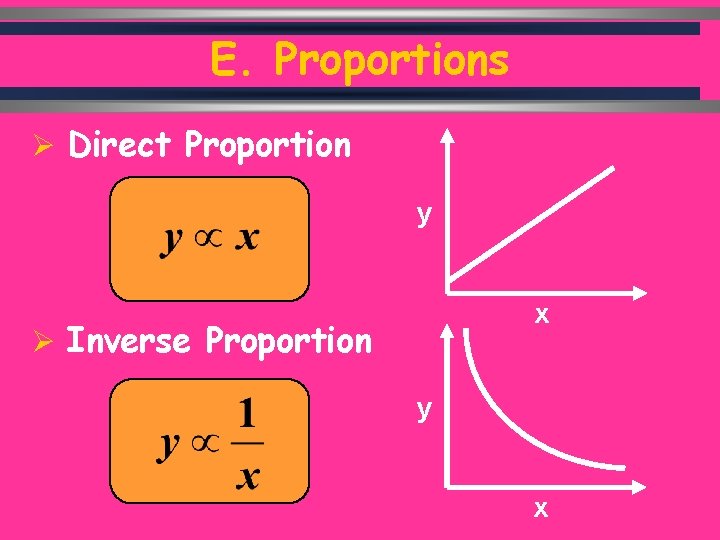
E. Proportions Ø Direct Proportion y x Ø Inverse Proportion y C. Johannesson x