Measurement and Quantities Understand the International System of

- Slides: 20

Measurement and Quantities • • • Understand the International System of Units Give the units of measurement with symbols Perform calculations involving units conversion

Everyday measurements Distance that we travel Cold Weather

Quantities • In maths we use numbers, in chemistry we use quantities. • A quantity is described by a number and a unit. • E. g. a. 100 kg is a quantity, in this case mass b. 100 is the number c. kg is the unit

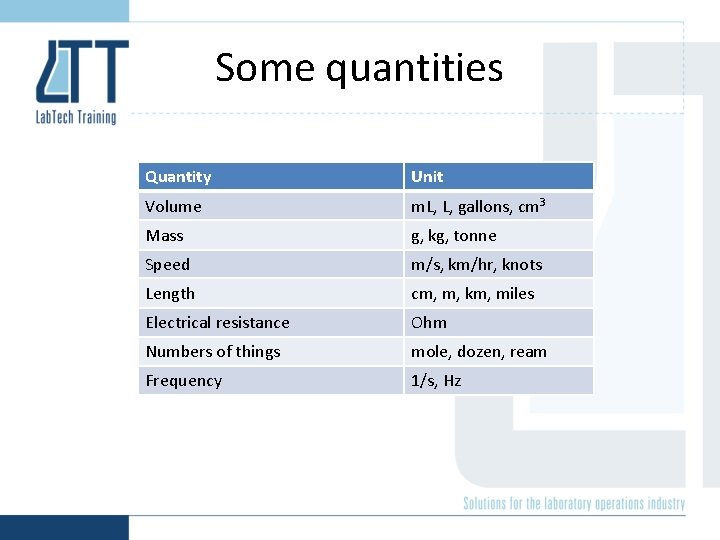
Some quantities Quantity Unit Volume m. L, L, gallons, cm 3 Mass g, kg, tonne Speed m/s, km/hr, knots Length cm, m, km, miles Electrical resistance Ohm Numbers of things mole, dozen, ream Frequency 1/s, Hz

SI Units • In 1960 the Conference Generale des Poids et Mesures, which is the international authority on metric system, accepted a universal, practical system of units and gave it the name “Le Systeme International d’Unites” with the abbreviation SI. By the 1970’s more than 20 countries had adopted the SI system.

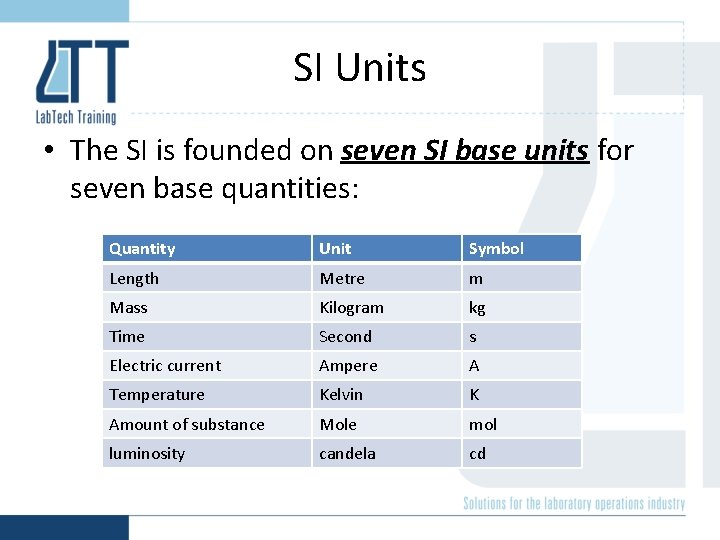
SI Units • The SI is founded on seven SI base units for seven base quantities: Quantity Unit Symbol Length Metre m Mass Kilogram kg Time Second s Electric current Ampere A Temperature Kelvin K Amount of substance Mole mol luminosity candela cd

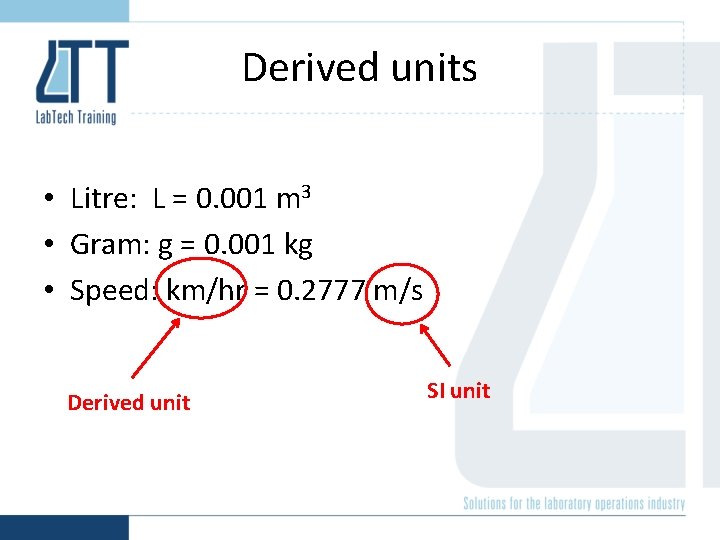
Derived units • Litre: L = 0. 001 m 3 • Gram: g = 0. 001 kg • Speed: km/hr = 0. 2777 m/s Derived unit SI unit

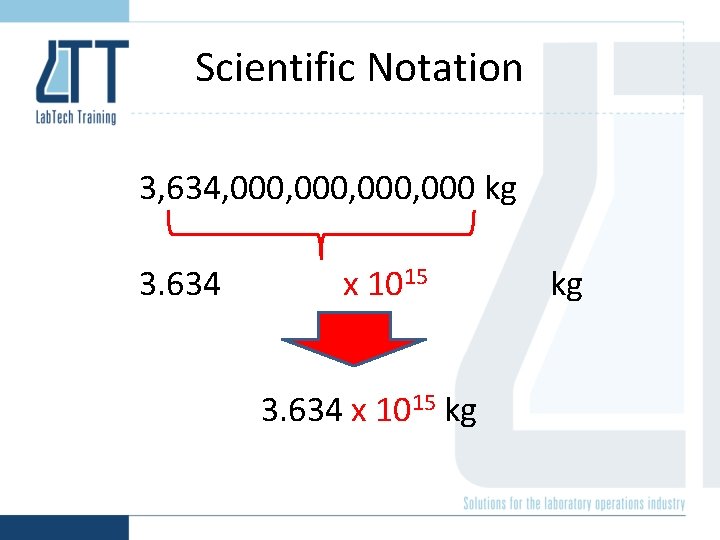
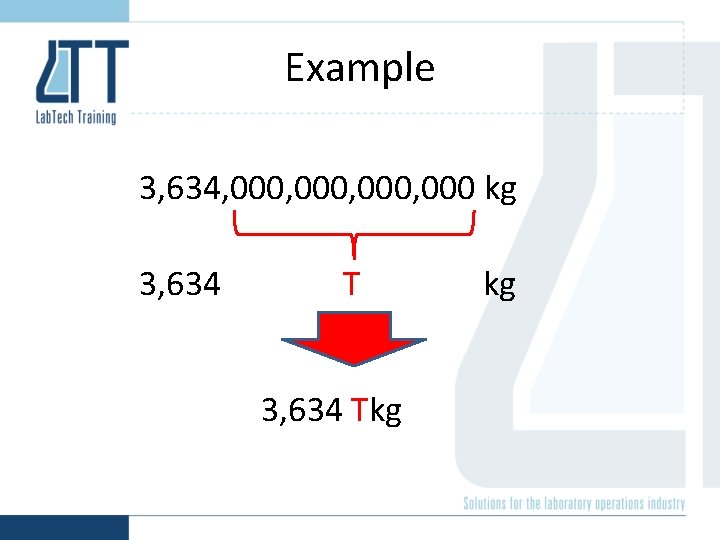
Scientific Notation 3, 634, 000, 000 kg 3. 634 x 1015 kg kg

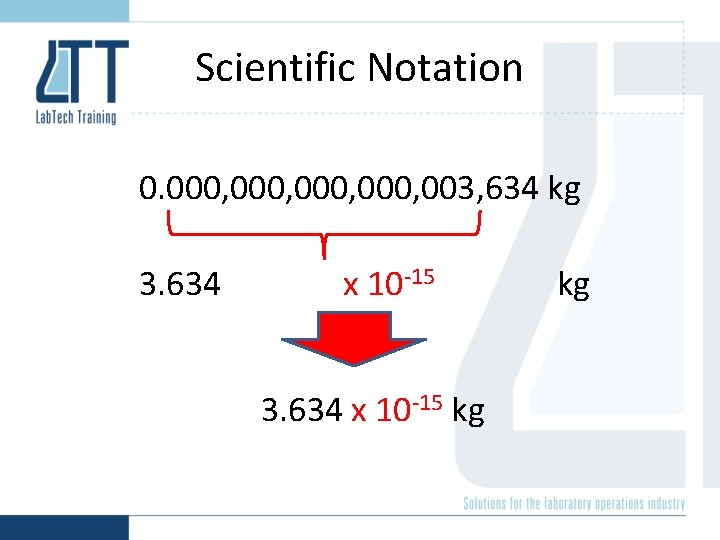
Scientific Notation 0. 000, 003, 634 kg 3. 634 x 10 -15 kg kg

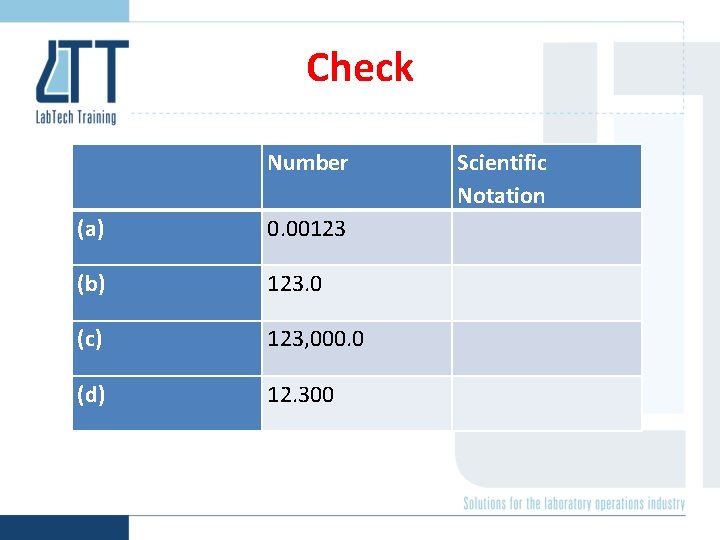
Check Number (a) 0. 00123 (b) 123. 0 (c) 123, 000. 0 (d) 12. 300 Scientific Notation

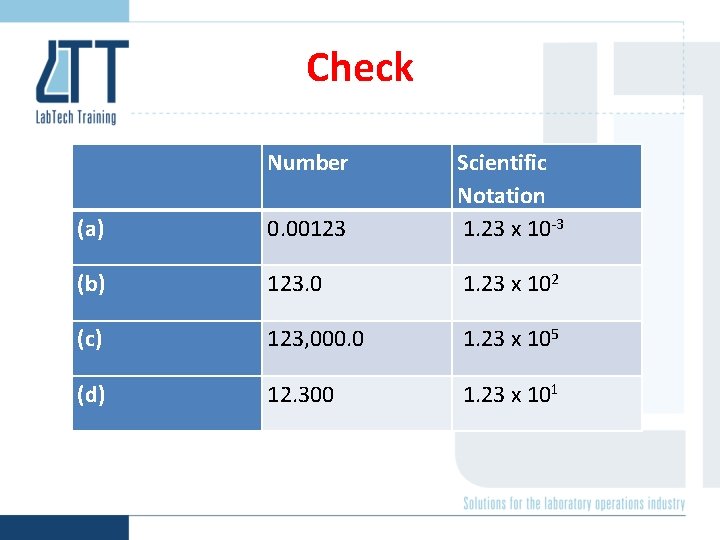
Check Number (a) 0. 00123 Scientific Notation 1. 23 x 10 -3 (b) 123. 0 1. 23 x 102 (c) 123, 000. 0 1. 23 x 105 (d) 12. 300 1. 23 x 101

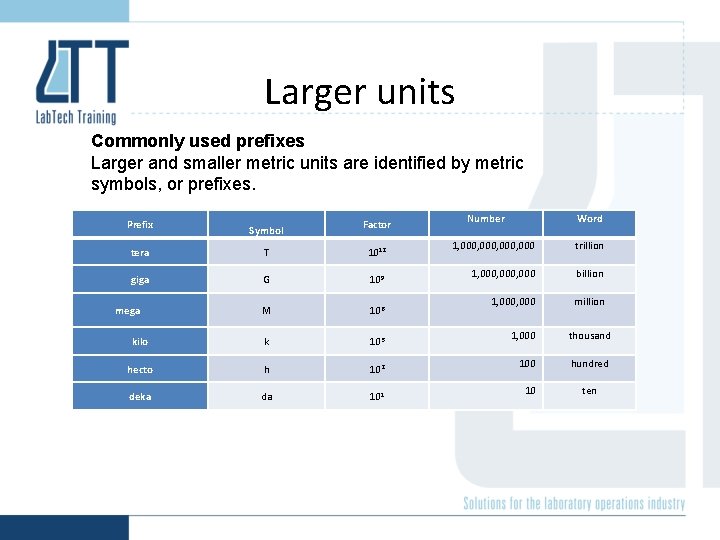
Larger units Commonly used prefixes Larger and smaller metric units are identified by metric symbols, or prefixes. Prefix Symbol Factor tera T 1012 giga G 109 M 106 kilo k 103 hecto h 102 deka da 101 mega Number Word 1, 000, 000 trillion 1, 000, 000 billion 1, 000 million 1, 000 thousand 100 hundred 10 ten

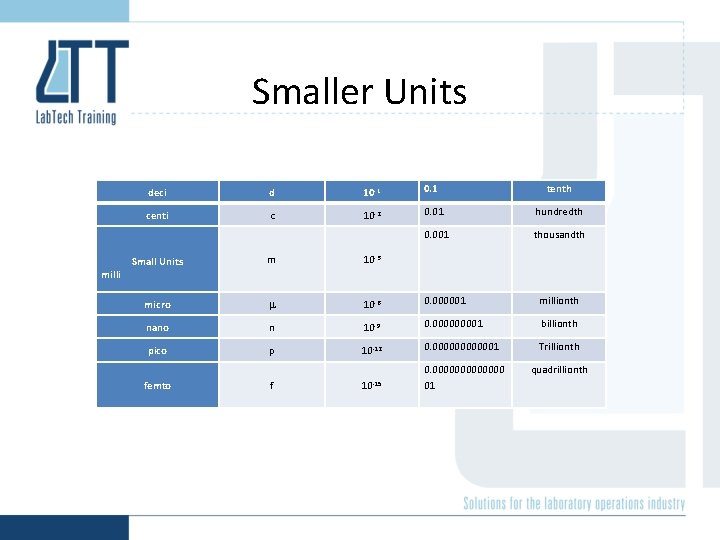
Smaller Units milli deci d 10 -1 0. 1 tenth centi c 10 -2 0. 01 hundredth 0. 001 thousandth Small Units m 10 -3 micro 10 -6 0. 000001 millionth nano n 10 -9 0. 00001 billionth pico p 10 -12 0. 0000001 Trillionth f 0. 0000000 01 quadrillionth 10 -15 femto

Example 3, 634, 000, 000 kg 3, 634 Tkg kg

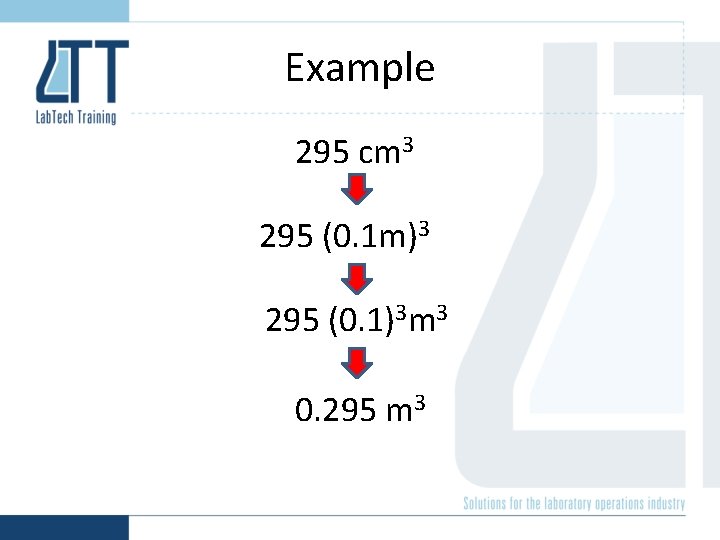
Example 295 cm 3 295 (0. 1 m)3 295 (0. 1)3 m 3 0. 295 m 3

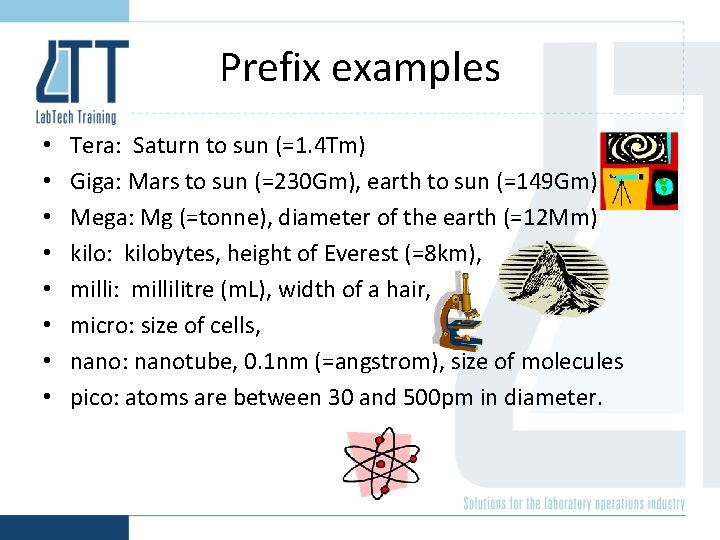
Prefix examples • • Tera: Saturn to sun (=1. 4 Tm) Giga: Mars to sun (=230 Gm), earth to sun (=149 Gm) Mega: Mg (=tonne), diameter of the earth (=12 Mm) kilo: kilobytes, height of Everest (=8 km), milli: millilitre (m. L), width of a hair, micro: size of cells, nano: nanotube, 0. 1 nm (=angstrom), size of molecules pico: atoms are between 30 and 500 pm in diameter.

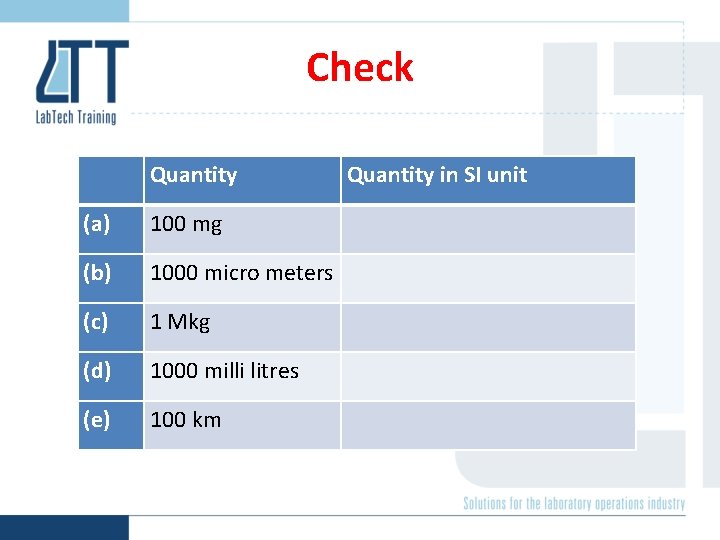
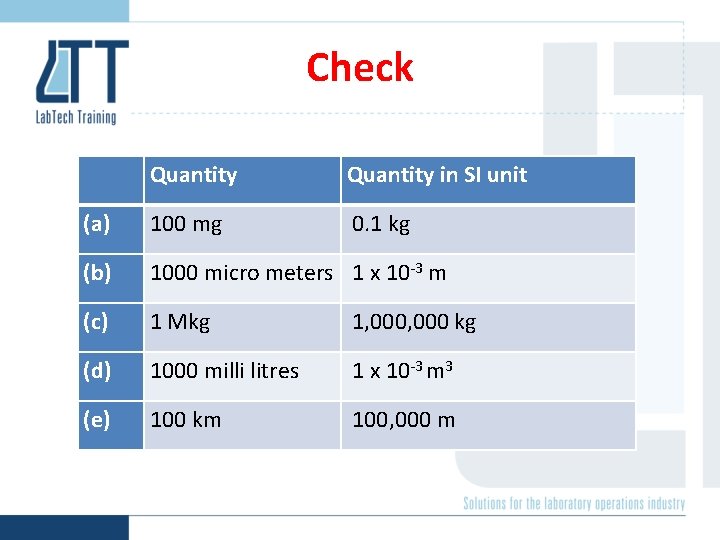
Check Quantity (a) 100 mg (b) 1000 micro meters (c) 1 Mkg (d) 1000 milli litres (e) 100 km Quantity in SI unit

Check Quantity in SI unit (a) 100 mg 0. 1 kg (b) 1000 micro meters 1 x 10 -3 m (c) 1 Mkg 1, 000 kg (d) 1000 milli litres 1 x 10 -3 m 3 (e) 100 km 100, 000 m

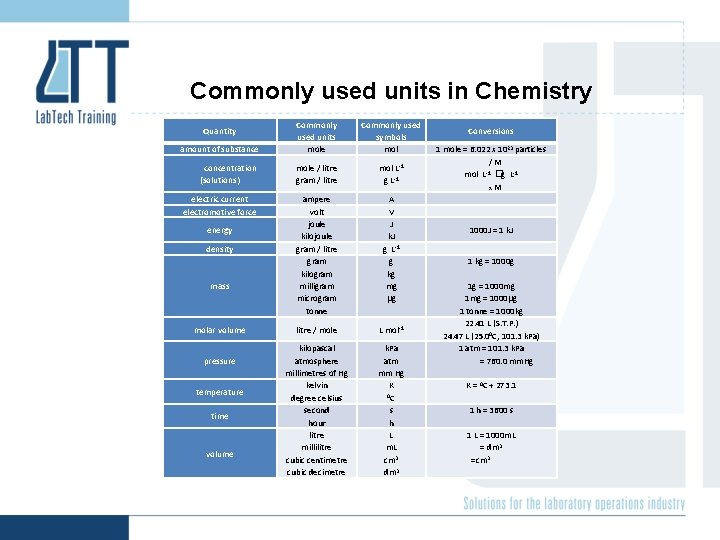
Commonly used units in Chemistry amount of substance Commonly used units mole Commonly used symbols mol concentration (solutions) mole / litre gram / litre mol L-1 g L-1 electric current electromotive force ampere volt joule kilojoule gram / litre gram kilogram milligram microgram tonne A V J k. J g L-1 g kg mg g litre / mole L mol-1 kilopascal atmosphere millimetres of Hg kelvin degree celsius second hour litre millilitre cubic centimetre cubic decimetre k. Pa atm mm Hg K 0 C s h L m. L cm 3 dm 3 Quantity energy density mass molar volume pressure temperature time volume Conversions 1 mole = 6. 022 x 1023 particles /M mol L-1 �g L-1 x. M 1000 J = 1 k. J 1 kg = 1000 g 1 g = 1000 mg 1 mg = 1000 g 1 tonne = 1000 kg 22. 41 L (S. T. P. ) 24. 47 L (25. 00 C, 101. 3 k. Pa) 1 atm = 101. 3 k. Pa = 760. 0 mm. Hg K = 0 C + 273. 1 1 h = 3600 s 1 L = 1000 m. L = dm 3 = cm 3

Summary • Quantity = number + unit • SI units • Larger units, smaller units