MDEpi Net Womens Health Technologies CRN Overview of








- Slides: 13

MDEpi. Net Women’s Health Technologies CRN: Overview of Objectives and Workstreams September 7, 2018 Courtney Baird, MS

Outline 1. Review of WHT-CRN Project Objectives 2. Key WHT-CRN Project Deliverables 3. WHT-CRN Federal Partners and their Roles 4. Timeline of Key Activities 5. Overview of Process used to Identify the Core Minimum Data Elements 2

Overall Project Objectives • Funding granted from the Patient-Centered Outcomes Research Trust Fund (PCORTF) • Help establish a strategically coordinated registry network (CRN) for women’s health technologies and develop tools to facilitate collection of data within the existing and new registries by leveraging clinical care data 3

Overall Project Objectives • Demonstrate that data in these registries can be used to do the following: • Evaluate the effectiveness, quality of life and safety associated with differing treatment options • Assess the effectiveness and quality of life associated with varying treatment options • Provide a framework for clinical studies to be conducted within the registry, including industry-sponsored studies required to fulfill the FDA’s request for pre-market and post-market regulatory activities • Allow healthcare providers to track surgeon volume, patient outcomes, and quality measures for quality improvement activities and fulfill upcoming Centers for Medicaid and Medicare Services (CMS), Physician Quality and Reporting Systems (PQRS) and maintenance of certification requirements 4

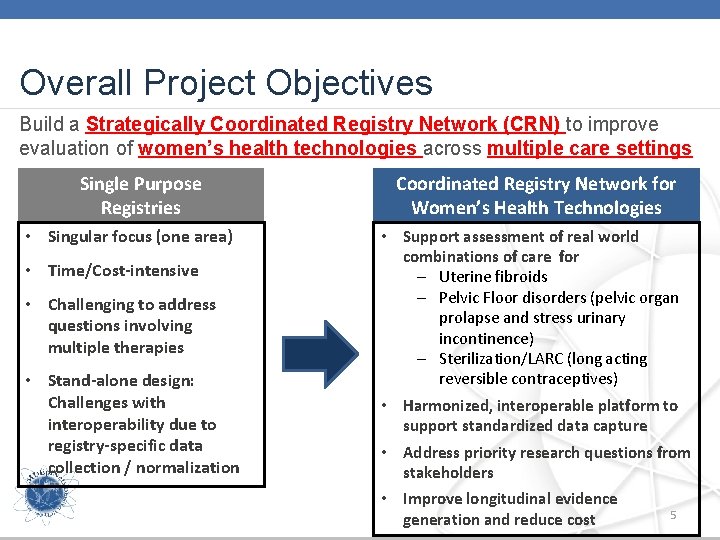
Overall Project Objectives Build a Strategically Coordinated Registry Network (CRN) to improve evaluation of women’s health technologies across multiple care settings Single Purpose Registries • Singular focus (one area) • Time/Cost-intensive • Challenging to address questions involving multiple therapies • Stand-alone design: Challenges with interoperability due to registry-specific data collection / normalization Coordinated Registry Network for Women’s Health Technologies • Support assessment of real world combinations of care for – Uterine fibroids – Pelvic Floor disorders (pelvic organ prolapse and stress urinary incontinence) – Sterilization/LARC (long acting reversible contraceptives) • Harmonized, interoperable platform to support standardized data capture • Address priority research questions from stakeholders • Improve longitudinal evidence generation and reduce cost 5

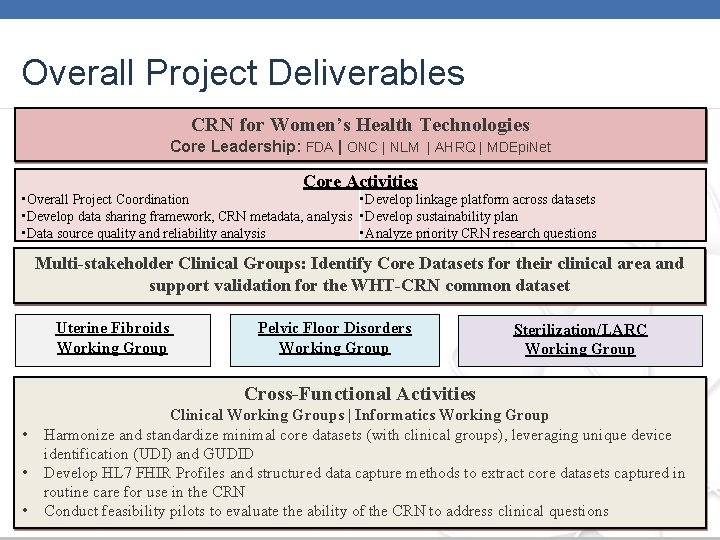
Overall Project Deliverables CRN for Women’s Health Technologies Core Leadership: FDA | ONC | NLM | AHRQ | MDEpi. Net Core Activities • Overall Project Coordination • Develop linkage platform across datasets • Develop data sharing framework, CRN metadata, analysis • Develop sustainability plan • Data source quality and reliability analysis • Analyze priority CRN research questions Multi-stakeholder Clinical Groups: Identify Core Datasets for their clinical area and support validation for the WHT-CRN common dataset Uterine Fibroids Working Group Pelvic Floor Disorders Working Group Sterilization/LARC Working Group Cross-Functional Activities • • • Clinical Working Groups | Informatics Working Group Harmonize and standardize minimal core datasets (with clinical groups), leveraging unique device identification (UDI) and GUDID Develop HL 7 FHIR Profiles and structured data capture methods to extract core datasets captured in routine care for use in the CRN Conduct feasibility pilots to evaluate the ability of the CRN to address clinical questions 6

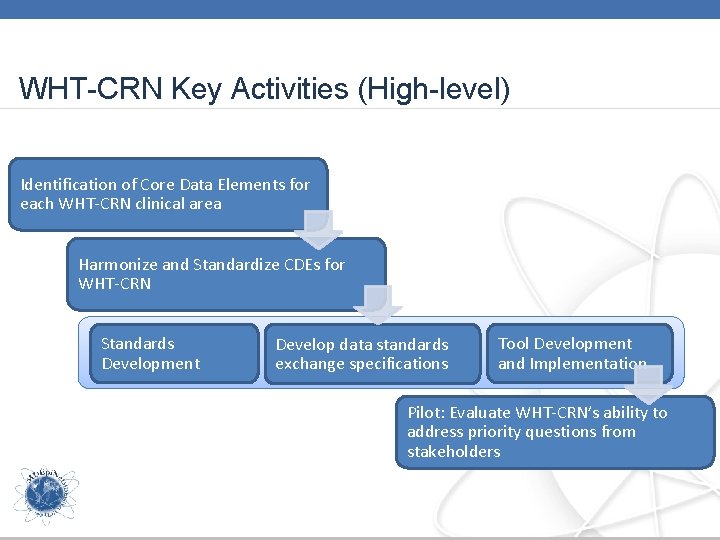
WHT-CRN Key Activities (High-level) Identification of Core Data Elements for each WHT-CRN clinical area Harmonize and Standardize CDEs for WHT-CRN Standards Development Develop data standards exchange specifications Tool Development and Implementation Pilot: Evaluate WHT-CRN’s ability to address priority questions from stakeholders

WHT-CRN Key Activities (High-level) Identification of Core Data Elements for each WHT-CRN clinical area CWG Session Focus: Each clinical working group (CWG) will present their core data elements, goals for how the dataset will be used, and next steps. Harmonize and Standardize CDEs for WHT-CRN Standards Development Develop data standards exchange specifications Tool Development and Implementation Pilot: Evaluate WHT-CRN’s ability to address priority questions from stakeholders

Process used to Identify the Core Minimum Data Elements For each clinical working group: • A group of experts was convened and selected from professional society leaders, the FDA, academia and industry. • An initial set of data elements was created from a review of the literature, regulatory requirements, and existing research efforts. • A formal 2 -3 round Delphi Method was employed to narrow down the list and achieve consensus on a core minimum dataset. 9

Delphi Process Overview • Challenges with a traditional consensus panel approach: • One person with strong personality can have a large effect on the decision • Lack of anonymity may introduce bias • Delphi Method • The Delphi process was developed to achieve consensus while minimizing bias from group dynamics and face-to-face responses • Group input is received through anonymous surveys • Provides an opportunity for group members to introduce new options and suggestions in between rounds • Results are analyzed to identify responses with strong consensus (e. g. >70%). • Options that lack consensus automatically dropped (<50%) • Multiple rounds until consensus achieved 10

Delphi Process Overview 11

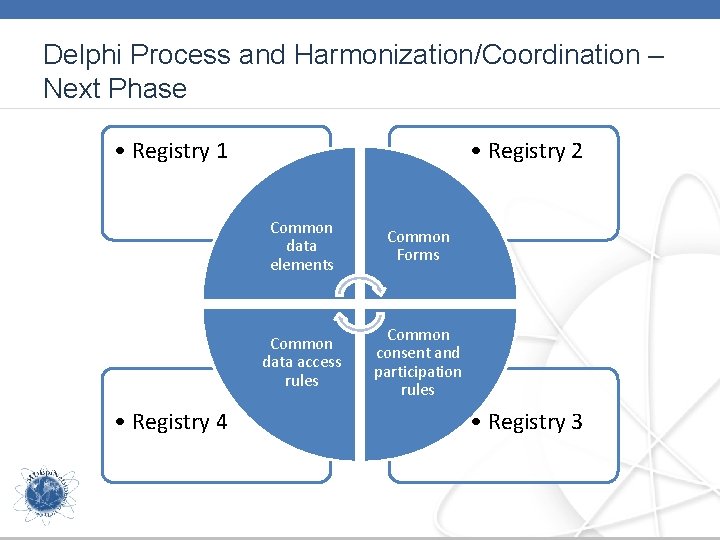
Delphi Process and Harmonization/Coordination – Next Phase • Registry 1 • Registry 4 • Registry 2 Common data elements Common Forms Common data access rules Common consent and participation rules • Registry 3

Thank you.
























