MCIT Research Data Core MCIT Research Data Core























- Slides: 32

MCIT Research - Data. Core MCIT Research -- Data. Core Alexander Bragat, Director – MCIT Research Data. Core 19 -SEP-2018

Outline • • • 2 Introduction Data. Core Organization Services Tools Data Management for Clinical Trials

Background Presentation Title Goes Here 3

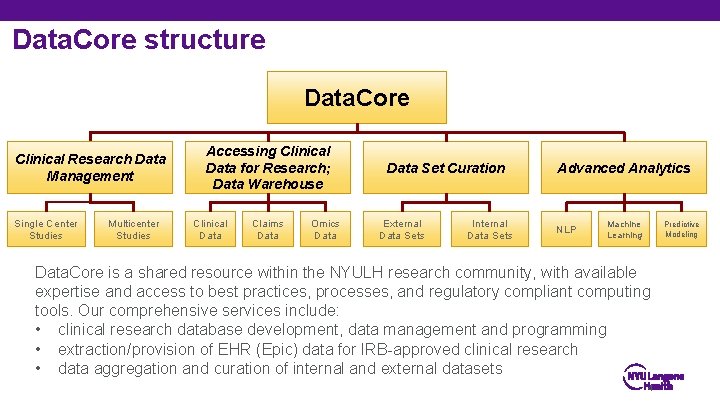
Data. Core structure Data. Core Clinical Research Data Management Single Center Studies Multicenter Studies Accessing Clinical Data for Research; Data Warehouse Clinical Data Claims Data Omics Data Set Curation External Data Sets Internal Data Sets Advanced Analytics NLP Machine Learning Predictive Modeling Data. Core is a shared resource within the NYULH research community, with available expertise and access to best practices, processes, and regulatory compliant computing tools. Our comprehensive services include: • clinical research database development, data management and programming • extraction/provision of EHR (Epic) data for IRB-approved clinical research • data aggregation and curation of internal and external datasets 4

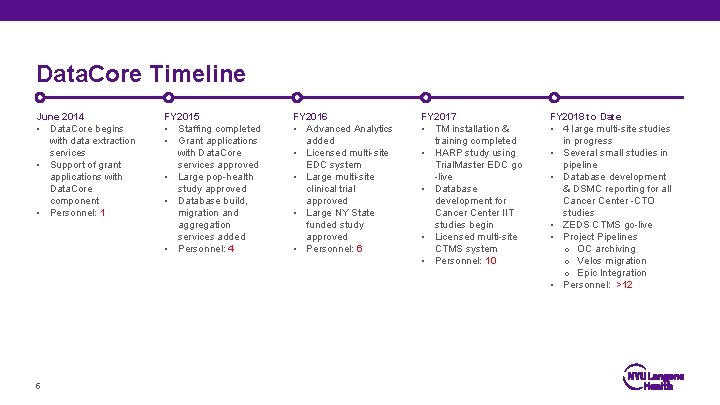
Data. Core Timeline June 2014 • Data. Core begins with data extraction services • Support of grant applications with Data. Core component • Personnel: 1 5 FY 2015 • Staffing completed • Grant applications with Data. Core services approved • Large pop-health study approved • Database build, migration and aggregation services added • Personnel: 4 FY 2016 • Advanced Analytics added • Licensed multi-site EDC system • Large multi-site clinical trial approved • Large NY State funded study approved • Personnel: 6 FY 2017 • TM installation & training completed • HARP study using Trial. Master EDC go -live • Database development for Cancer Center IIT studies begin • Licensed multi-site CTMS system • Personnel: 10 FY 2018 to Date • 4 large multi-site studies in progress • Several small studies in pipeline • Database development & DSMC reporting for all Cancer Center -CTO studies • ZEDS CTMS go-live • Project Pipelines o OC archiving o Velos migration o Epic Integration • Personnel: >12

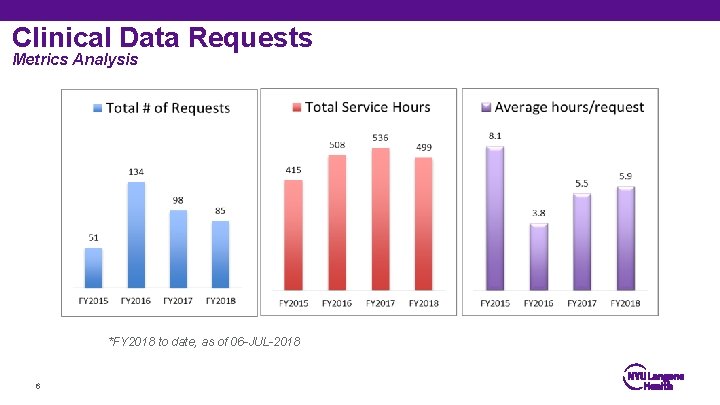
Clinical Data Requests Metrics Analysis *FY 2018 to date, as of 06 -JUL-2018 6

Large awarded grants with Data. Core collaboration Long-term Suppressive Valacyclovir Treatment for Herpes Zoster Opthalmicus

Data. Core Services Presentation Title Goes Here 8

Clinical Research Data Flow - Schematic 9

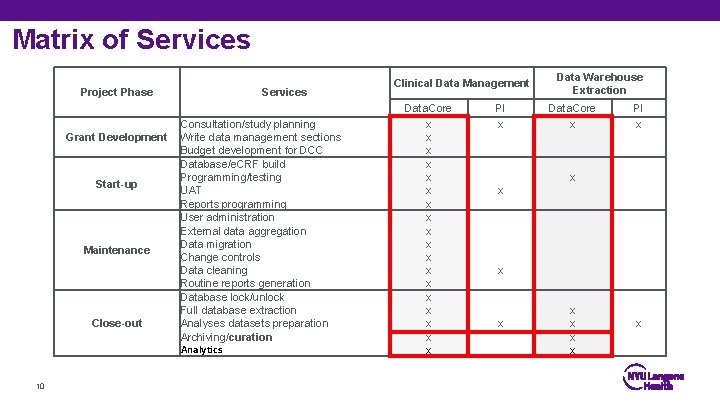
Matrix of Services Project Phase Grant Development Start-up Maintenance Close-out 10 Services Consultation/study planning Write data management sections Budget development for DCC Database/e. CRF build Programming/testing UAT Reports programming User administration External data aggregation Data migration Change controls Data cleaning Routine reports generation Database lock/unlock Full database extraction Analyses datasets preparation Archiving/curation Analytics Clinical Data Management Data. Core x x x x x PI x Data Warehouse Extraction Data. Core x PI x x x x x

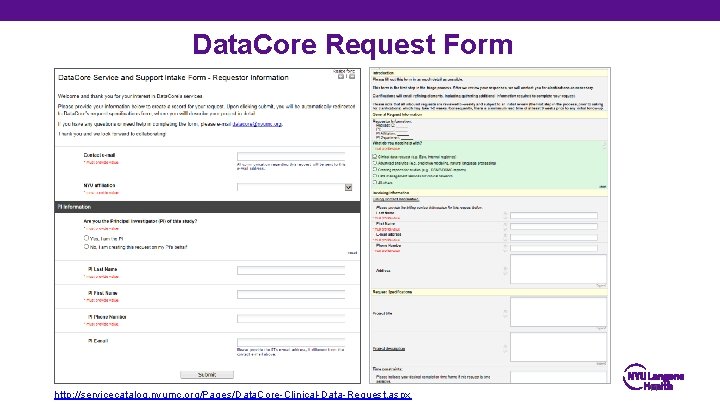
Data. Core Request Form http: //servicecatalog. nyumc. org/Pages/Data. Core-Clinical-Data-Request. aspx 11

Data. Core Service Fees • Data extraction services are currently charged $75/hr of analyst time – medical students & trainees that are primary investigators get 10 hrs free • Database development & Clinical Data Management fees are directly based on %FTE required and corresponding salaries – REDCap database development can be requested using the $75/hr rate as OTPS – Assistance is provided during grant preparation subject to sponsor requirements & availability – License fee for Trial. Master EDC is currently free • NOTE: A consolidated Data. Core/MCIT service fee is being considered to update the current rate 12

Data. Core Toolbox Presentation Title Goes Here 13

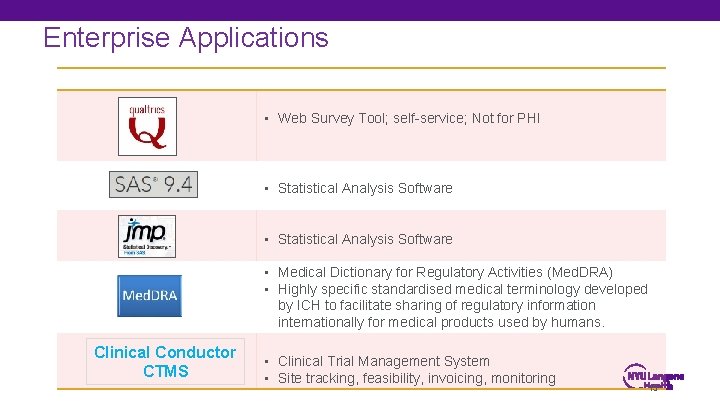
Enterprise Applications • Electronic data capture (EDC) for large, regulated, multi-site trials and investigator-initiated trials • 21 CFR Part 11 compliant; e. Source interface with Epic • Self-service EDC for investigators; ideal for small studies • Data. Core support available for e. CRF development and migration of legacy study data into compliant system • Data. Core support to extract clinical data from Epic for research • Self-service cohort identification tool for investigators • Access to CDISC Shared Health and Research Electronic Library (SHARE) • Industry standards in clinical data interchange 14

Enterprise Applications • Web Survey Tool; self-service; Not for PHI • Statistical Analysis Software • Medical Dictionary for Regulatory Activities (Med. DRA) • Highly specific standardised medical terminology developed by ICH to facilitate sharing of regulatory information internationally for medical products used by humans. Clinical Conductor CTMS • Clinical Trial Management System • Site tracking, feasibility, invoicing, monitoring 15

Self-service Tools i 2 b 2: Informatics for Integrating Biology & the Bedside • • >11, 000 queries 140+ active users ACT network will allow national scale queries MCIT service catalog to obtain an account https: //servicecatalog. nyumc. org/pages/Application-Access-or-Enhancement. aspx 16

Why use any of this stuff (EDC) ? • Because we back up your data • Because we provide it at no or subsidized cost • Because you have obligations to protect the confidentiality, security and integrity of data relating to human subjects - Health Insurance Portability and Accountability Act (HIPAA) FDA regulations Sponsor requirements IRB requirements Medical Center policies • Because you will want your electronic data to be auditable if you are ever accused of manipulating data - Google docs, Excel, Access, Filemaker Pro do not maintain audit trails and should never be used for non-exempt research 17 *** Don’t put the integrity of your data or your reputation at risk! ***

Why use any of this stuff? (Continued) • EDCs are created to provide secure access to your data -- anywhere, anytime • NEVER store research data containing PHI on an unencrypted or personal computer or portable storage devices such as USB drives or external hard drives - Remember, password protection does not always equal encryption! - If absolutely necessary to use a portable device, use only an Iron. Key (encrypted USB drive) provided by MCIT • Stolen laptops containing PHI of research subjects have resulted in large fines - March 2016: $3. 9 M Feinstein Medical Research Institute (Northwell) - Laptop stolen from employee’s car contains e. PHI of 13, 000 subjects and patients - September 2012: $1. 5 M Mass Eye and Ear - Laptop is either stolen or lost, contains e. PHI of 3, 500 subjects and patients 18 ** Don’t put the integrity of your data or your reputation at risk! ***

Data. Core and Data Managment: Alexander Bragat, PMP Alexander. Bragat@nyumc. org REDCap: Rick Church Richard. Church@nyumc. org Data Requests: Ed Iturrate, MD, MSW, FHM Eduardo. Iturrate@nyumc. org 19

THANK YOU

What is Clinical Data Management? Presentation Title Goes Here 21

What is a CRF? What is GCP? What is a protocol? 1. 11 Case report form (CRF): A printed, optical, or electronic document designed to record all of protocol-required information the protocol-required information to be reported to the sponsor on each trial subject. 1. 24 Good Clinical Practice (GCP): A standard for the design, conduct, performance, monitoring, auditing, recording, analyses, and reporting of clinical trials that provides assurance that the data and reported results are credible and accurate, and that the rights, integrity, and confidentiality of trial subjects are protected. 1. 44 Protocol: A document that describes the objective(s), design, methodology, statistical considerations, and organization of a trial. The protocol usually also gives the background and rationale for the trial, but these could be provided in other protocol referenced documents. Throughout the ICH GCP Guidance, the term protocol refers to protocol and protocol amendments. Reference: US DHHS, FDA, CDER, CBER. (1996). Guidance for industry: E 6 Good clinical practice: Consolidated guidance. Retrieved from http: //www. fda. gov/downloads/Drugs/. . . /Guidances/ucm 073122. pdf 22

What are the investigators’ data responsibilities ? 4. 9. 1 The investigator should ensure the accuracy, completeness, legibility, and timeliness of the data reported to the sponsor in the CRFs and in all required reports. 4. 9. 2 Data reported on the CRF, which are derived from source documents, should be consistent with the source documents or the discrepancies should be explained. 4. 9. 3 Any change or correction to a CRF should be dated, initialed, and explained (if necessary) and should not obscure the original entry (i. e. , an audit trail should be maintained); this applies to both written and electronic changes or corrections. . . The investigator should retain records of the changes and corrections. Reference: US DHHS, FDA, CDER, CBER. (1996). Guidance for industry: E 6 Good clinical practice: Consolidated guidance. Retrieved from http: //www. fda. gov/downloads/Drugs/. . . /Guidances/ucm 073122. pdf 23

What is ALCOA ? Data that is: • Attributable to the person generating the data • Legible, readable and permanent • Contemporaneous recording or logging of results, as they happen, as per the protocol • Original • Accurate 24

What is clinical data management (CDM) ? The processes and tools implemented to ensure and facilitate the timely collection of complete and accurate protocol-required information on each trial subject in order to provide traceable data for statistical analyses that provide credible results. 25

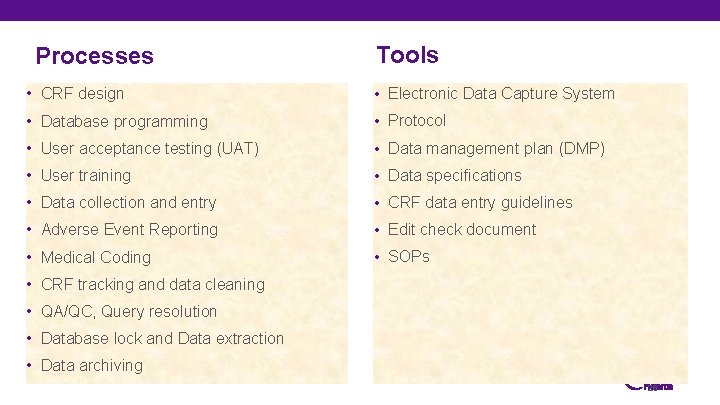
Processes Tools • CRF design • Electronic Data Capture System • Database programming • Protocol • User acceptance testing (UAT) • Data management plan (DMP) • User training • Data specifications • Data collection and entry • CRF data entry guidelines • Adverse Event Reporting • Edit check document • Medical Coding • SOPs • CRF tracking and data cleaning • QA/QC, Query resolution • Database lock and Data extraction • Data archiving 26

Roles and responsibilities Team collaboration • CDM – process oversight • Data Management • EDC developer – database build • Clinical personnel • CRA – data entry • Clinical operations • Monitor – source data verification • Biostatistics • Coders – coding of AE/CM • Technology vendors • QA/QC – data validation • Research IT • PI – e. CRF sign-off • IXRS/Supplies vendors 27

Study design considerations • Clinical study type - Randomized controlled clinical trial (RCT) - Placebo or active control, blinding, phase (1 -4) - Clinical pharmacology studies (SAD, MAD, BE, etc. ) • Experimental Design - Parallel, cross-over, double-cross-over, etc. • Statistical Analyses - Superiority, non-inferiority, bioequivalence 28

e. CRF design considerations • Matrix of data elements - Visit Schedule (list of assessments) - Primary and secondary endpoints, safety - Patient-reported outcomes • Data domains - e. g. DM, AE, CM, LB, MH, PE, VS, etc. • Data fields - Variables (continuous, categorical) - Type (character, numeric, date) 29 - Ranges (used for data validation checks)

Data Management Plan Documents the processes, tools, roles and deliverables: • Responsibility matrix, scope of work, communication plan • CRF design, study setup, data entry guidelines • Data flow, validation plan and data cleaning • External data management, reconciliation and lab ranges • AE/CM coding (MEDDRA, WHO-DD) • Reports, data extractions • Database locks, study closeout, archiving and data security Reference: Prokscha, S. (2007). Practical guide to clinical data management. Boca Raton, FL: CRC Press. 30

31

THANK YOU