Mc Murry Chapter 1 Structure Bonding Organic Chemistry
Mc. Murry Chapter 1 Structure & Bonding Organic Chemistry I S. Imbriglio

Organic Chemistry: What is it? • 1770: Organic chemistry referred to compounds isolated from living things. _____ belief that a “magic” force, present in plants and animals, is required to make organic compounds • 1789: Lavoisier observed that organic compounds are composed primarily of _____________
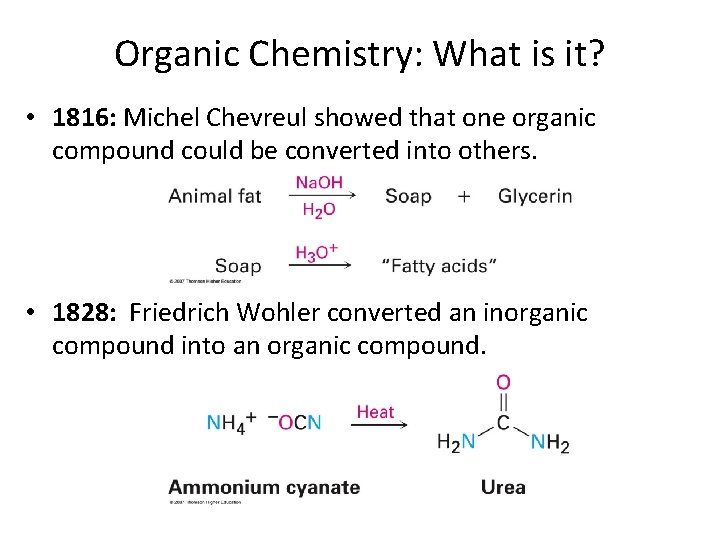
Organic Chemistry: What is it? • 1816: Michel Chevreul showed that one organic compound could be converted into others. • 1828: Friedrich Wohler converted an inorganic compound into an organic compound.
Organic Chemistry: the study of carbon compounds • ____of all known compounds are composed of carbon (~30 million known compounds) • ____of chemists define themselves as organic • Organic chemistry is crucial to our way of life: Pharmaceutical, Petroleum, Materials/Polymer, OUR BODIES!
Why Carbon? What makes carbon so special? (3 things)

Why Carbon? • Carbon atoms form _________ bonds to other atoms (including other carbon atoms). Incredible Structural Diversity!
Review of the Atomic Structure: – ______ charged nucleus – dense and small – ________ charged electrons in a cloud around the nucleus – Diameter approximately 2 10 -10 m

Review of the Atom Orbitals: - Three-dimensional shapes indicating where the electron is most likely to be found – s, p, d, f - We will focus on s and p orbitals – carbon atoms do not have d or f orbitals
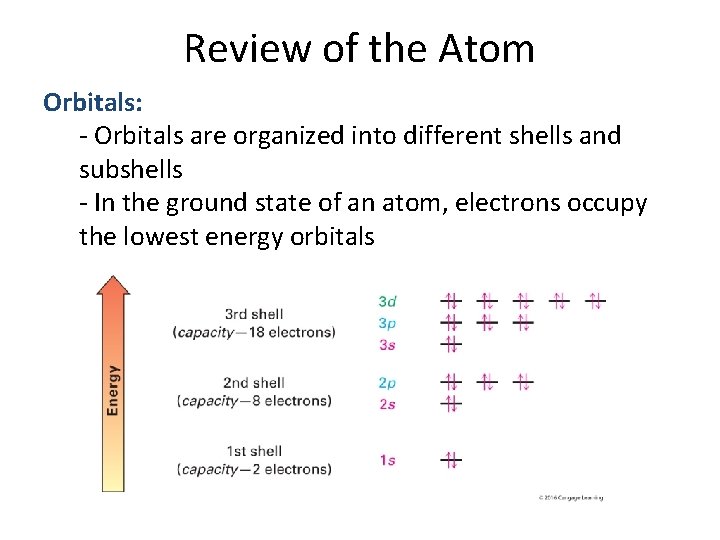
Review of the Atom Orbitals: - Orbitals are organized into different shells and subshells - In the ground state of an atom, electrons occupy the lowest energy orbitals
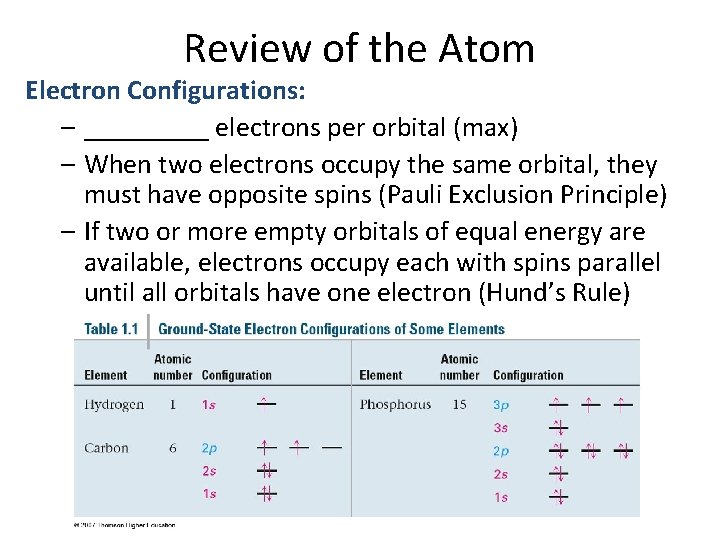
Review of the Atom Electron Configurations: – _____ electrons per orbital (max) – When two electrons occupy the same orbital, they must have opposite spins (Pauli Exclusion Principle) – If two or more empty orbitals of equal energy are available, electrons occupy each with spins parallel until all orbitals have one electron (Hund’s Rule)
• Write the electron configuration for oxygen. O
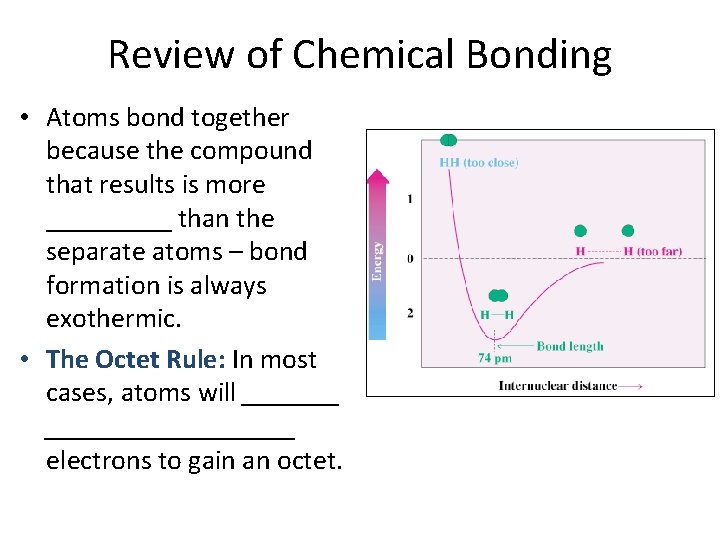
Review of Chemical Bonding • Atoms bond together because the compound that results is more _____ than the separate atoms – bond formation is always exothermic. • The Octet Rule: In most cases, atoms will _____________ electrons to gain an octet.

Review of Chemical Bonding • An _______ is the electrostatic attraction between a cation (____) and an anion (____). • The two bonded atoms do not share electrons.
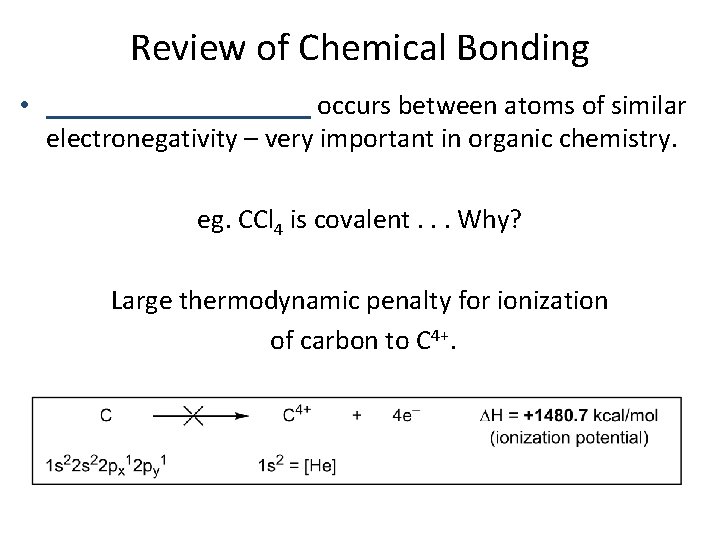
Review of Chemical Bonding • __________ occurs between atoms of similar electronegativity – very important in organic chemistry. eg. CCl 4 is covalent. . . Why? Large thermodynamic penalty for ionization of carbon to C 4+.

Review of Chemical Bonding • Instead of transferring electrons, each chlorine atom shares one valence electron with carbon so that every atom has a filled valence shell (an octet). Lone Pairs: ______________________
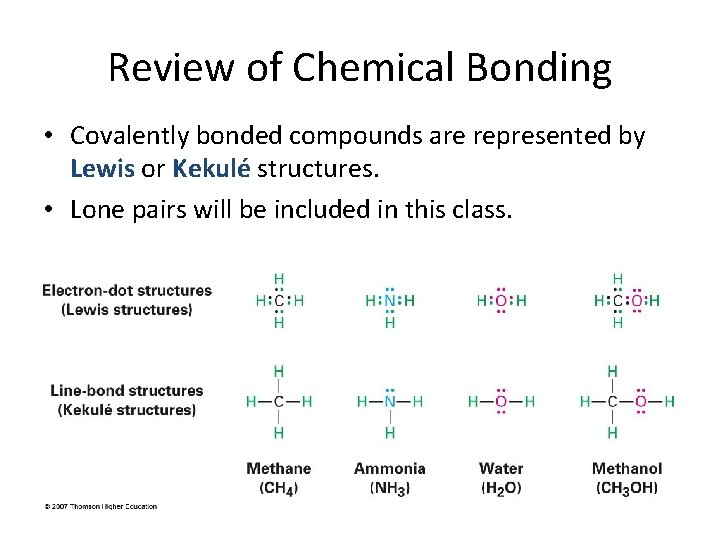
Review of Chemical Bonding • Covalently bonded compounds are represented by Lewis or Kekulé structures. • Lone pairs will be included in this class.

Review of Chemical Bonding • The number of covalent bonds that a main group element must form to achieve an octet equals eight minus its group number.

Keeping the common bonding patterns in mind, draw a valid Kekulé structure for each of the following formulas. Include all lone pairs. CH 3 NH 2 O 2 C 3 H 8

Review of Chemical Bonding Multiple Covalent Bonds • Atoms can share more than one electron pair to gain a full octet. – _________: two electron pairs shared between two atoms – _________: three electron pairs shared between two atoms
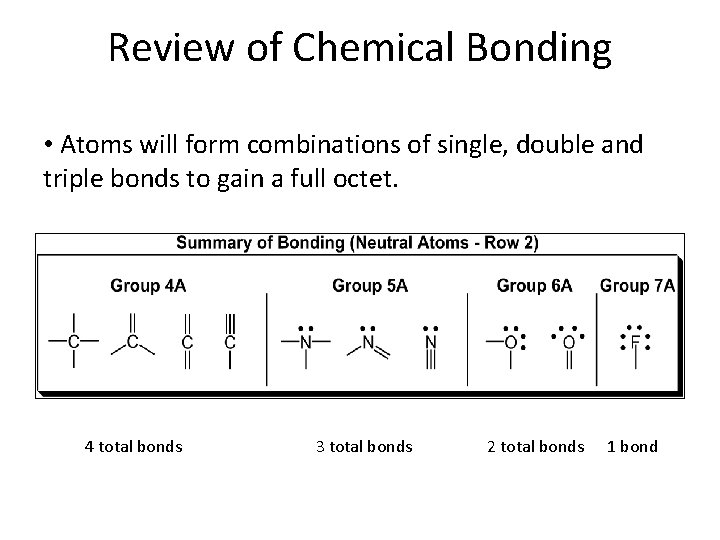
Review of Chemical Bonding • Atoms will form combinations of single, double and triple bonds to gain a full octet. 4 total bonds 3 total bonds 2 total bonds 1 bond

Draw a valid Kekulé structure for each of the following formulas. Include all lone pairs. CH 3 CO 2 H HCN
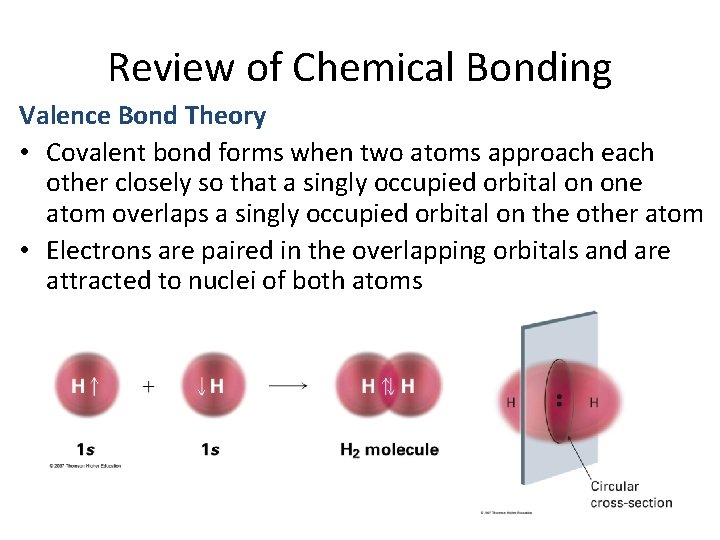
Review of Chemical Bonding Valence Bond Theory • Covalent bond forms when two atoms approach each other closely so that a singly occupied orbital on one atom overlaps a singly occupied orbital on the other atom • Electrons are paired in the overlapping orbitals and are attracted to nuclei of both atoms

Review of Chemical Bonding • Carbon uses its four valence electrons to form four covalent bonds. • Methane is a perfect tetrahedron – all of the C-H bonds are equivalent. • The four orbitals that make those bonds must also be equivalent.

Review of Chemical Bonding Hybridization • Atomic orbitals (s, p) on the same atom can be combined to form hybrid orbitals (sp, sp 2, sp 3) with geometries similar to those observed experimentally. • Hybrid Orbitals: – Are more directional (better bonding overlap with orbitals on other atoms) – Minimize electron-electron repulsion (think VSEPR)

Review of Chemical Bonding • sp 3 Hybridization: One s and three p orbitals combine to form four new sp 3 hybrid orbitals. The large lobes of the four sp 3 orbitals are pointed 109. 5 from each other – a tetrahedral geometry. back lobe large lobe

Review of Chemical Bonding sp 3 Hybridization in Methane • Each sp 3 orbital on carbon overlaps with a 1 s orbital on hydrogen to form four sigma bonds. • __________: cylindrically symmetrical bond resulting from head-on overlap of two orbitals along the bonding axis Orbital Picture Ball & Stick Model Space-Filling Model
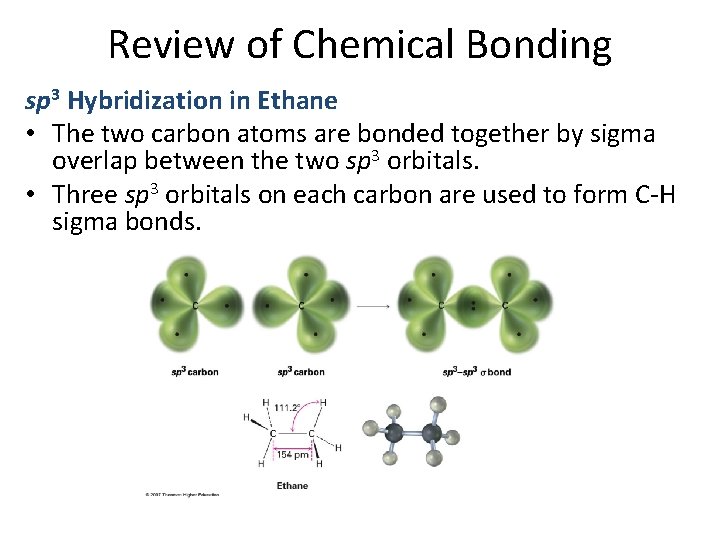
Review of Chemical Bonding sp 3 Hybridization in Ethane • The two carbon atoms are bonded together by sigma overlap between the two sp 3 orbitals. • Three sp 3 orbitals on each carbon are used to form C-H sigma bonds.

Review of Chemical Bonding • sp 2 Hybridization: One s orbital combines with two p orbitals to give three new sp 2 hybrid orbitals • One p orbital is left unhybridized. • The large lobes of the sp 2 orbitals are pointed 120 from each other – a trigonal planar geometry.

Review of Chemical Bonding sp 2 Hybridization in Ethylene • Two sp 2 orbitals overlap head-on to form a sigma bond – Electrons in the sigma bond are along the bonding axis • Two p orbitals overlap side-by-side to form a pi bond – Electrons in the pi bond occupy regions above and below the bonding axis (not cylindrically symmetrical)
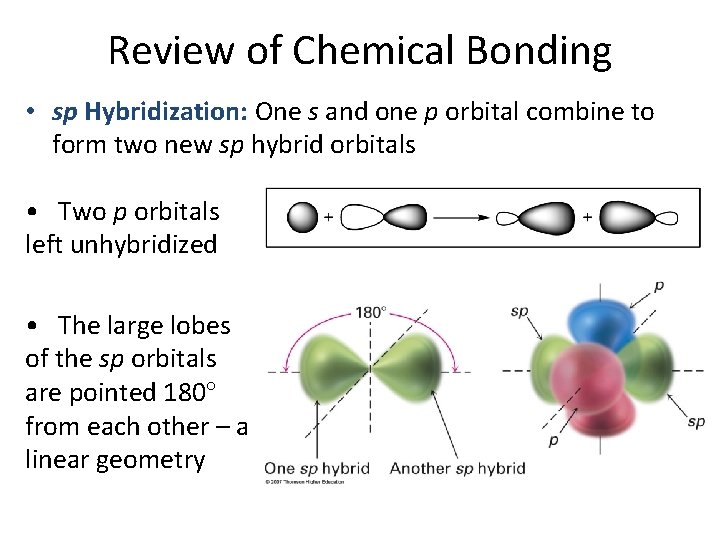
Review of Chemical Bonding • sp Hybridization: One s and one p orbital combine to form two new sp hybrid orbitals • Two p orbitals left unhybridized • The large lobes of the sp orbitals are pointed 180 from each other – a linear geometry

Review of Chemical Bonding sp Hybridization in Acetylene • Two sp orbitals overlap head-on to form a sigma bond • Two vertical p orbitals overlap side-by-side to form pi bond • Two horizontal p orbitals overlap side-by-side to form pi bond

Review of Chemical Bonding Assigning Hybridization to Atoms • The hybridization of an atom in a molecule can be determined by counting the number of hybrid orbitals the atom is using. • Hybrid orbitals are used to: – Form sigma bonds (not pi bonds) – Hold lone pairs
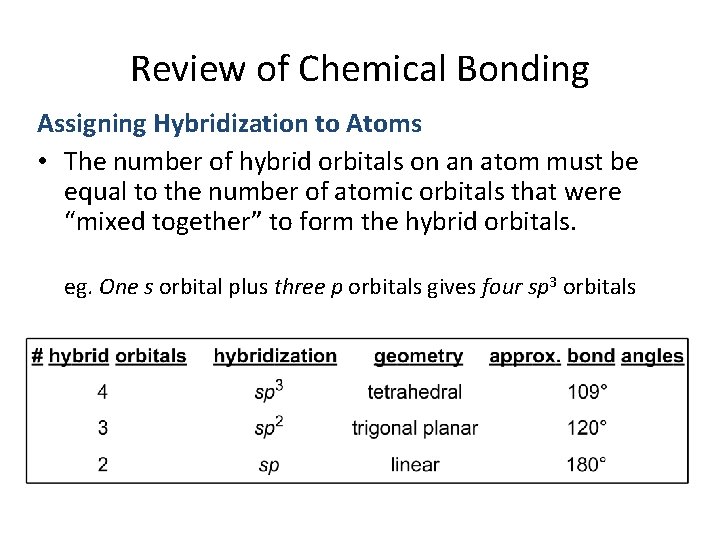
Review of Chemical Bonding Assigning Hybridization to Atoms • The number of hybrid orbitals on an atom must be equal to the number of atomic orbitals that were “mixed together” to form the hybrid orbitals. eg. One s orbital plus three p orbitals gives four sp 3 orbitals
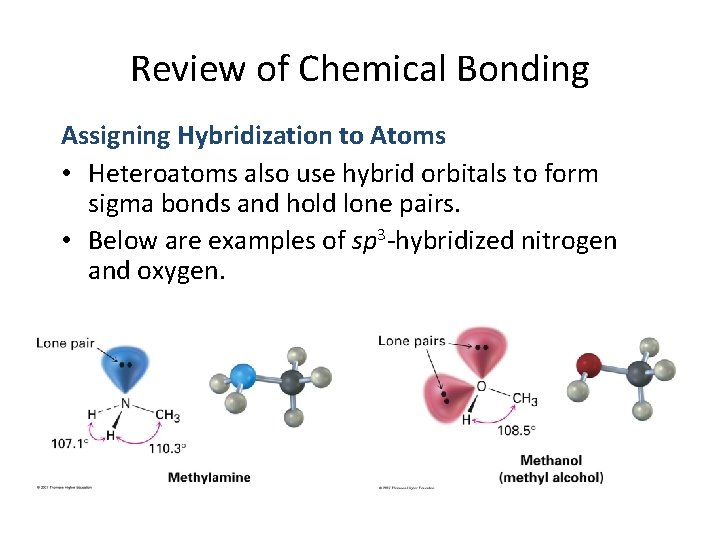
Review of Chemical Bonding Assigning Hybridization to Atoms • Heteroatoms also use hybrid orbitals to form sigma bonds and hold lone pairs. • Below are examples of sp 3 -hybridized nitrogen and oxygen.
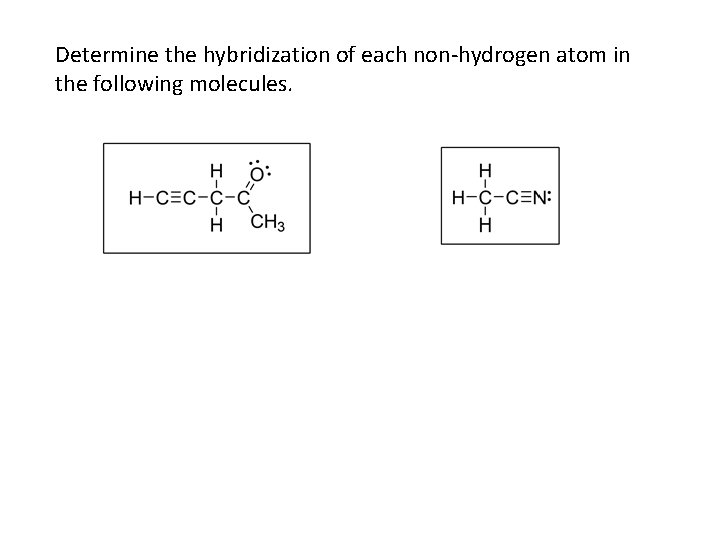
Determine the hybridization of each non-hydrogen atom in the following molecules.

Representing Molecules Condensed Structures

Representing Molecules • Lewis and Kekulé structures are only adequate for very small molecules.

Representing Molecules Skeletal Structures (Line-Angle Formulas) Rules for Drawing Skeletal Structures 1. Do not draw carbon atoms. A carbon atom is assumed to be at each intersection and at the end of each line. 2. Do not draw hydrogen atoms bonded to carbon. Assume enough C-H bonds to give each carbon a filled valence. 3. Draw all heteroatoms and attached hydrogens.

Representing Molecules
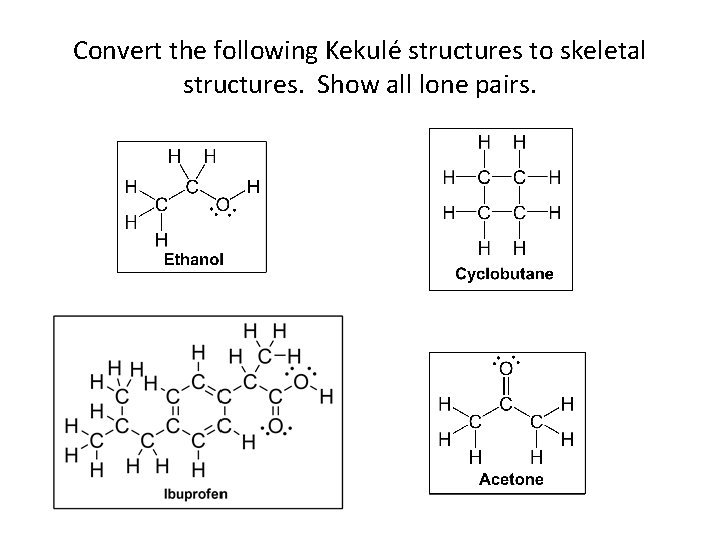
Convert the following Kekulé structures to skeletal structures. Show all lone pairs.
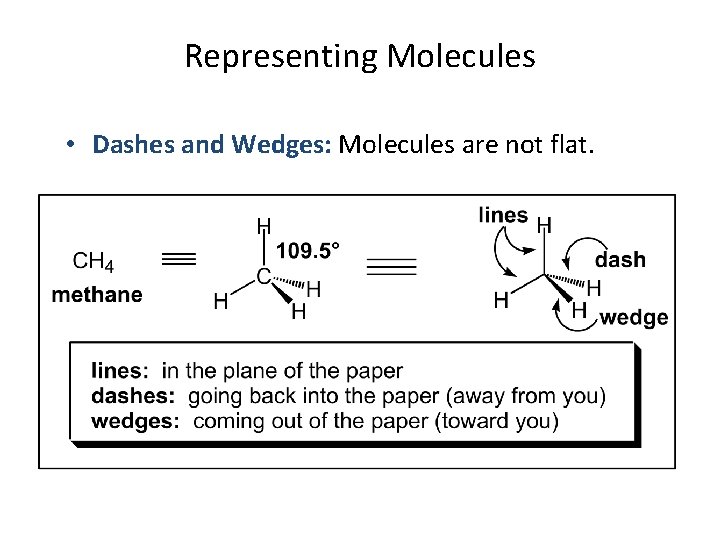
Representing Molecules • Dashes and Wedges: Molecules are not flat.
- Slides: 41