May 2009 doc IEEE 802 15 09 0435



- Slides: 7

May 2009 doc. : IEEE 802. 15 -09 -0435 -00 -0006 IEEE 802 Interim Meeting Montreal 12 th May 2009 802. 15. 6 - Review of Exempt Spectrum that may be of interest for medical use Andy Gowans, Spectrum Policy Group, Ofcom UK 14 th May 2009 Submission 0 Andrew Gowans

May 2009 doc. : IEEE 802. 15 -09 -0435 -00 -0006 Contents • European Exempt Bands – Dedicated Medical bands in EC Decision – General use SRD bands that can be used • This years review of EC Decision – Proposed Medical Bands – Other medical band proposal Submission 1 Andrew Gowans

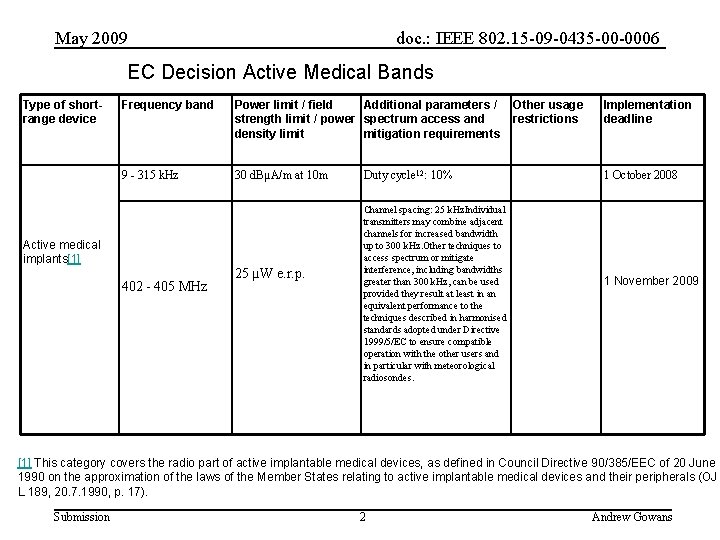
May 2009 doc. : IEEE 802. 15 -09 -0435 -00 -0006 EC Decision Active Medical Bands Type of shortrange device Frequency band Power limit / field Additional parameters / strength limit / power spectrum access and density limit mitigation requirements 9 - 315 k. Hz 30 d. BµA/m at 10 m Active medical implants[1] 402 - 405 MHz 25 µW e. r. p. Duty cycle 12: 10% Channel spacing: 25 k. Hz. Individual transmitters may combine adjacent channels for increased bandwidth up to 300 k. Hz. Other techniques to access spectrum or mitigate interference, including bandwidths greater than 300 k. Hz, can be used provided they result at least in an equivalent performance to the techniques described in harmonised standards adopted under Directive 1999/5/EC to ensure compatible operation with the other users and in particular with meteorological radiosondes. Other usage restrictions Implementation deadline 1 October 2008 1 November 2009 [1] This category covers the radio part of active implantable medical devices, as defined in Council Directive 90/385/EEC of 20 June 1990 on the approximation of the laws of the Member States relating to active implantable medical devices and their peripherals (OJ L 189, 20. 7. 1990, p. 17). Submission 2 Andrew Gowans

May 2009 doc. : IEEE 802. 15 -09 -0435 -00 -0006 EC Decision Generic SRD and Application Specific SRD Use See separate table in Word Document: https: //mentor. ieee. org/802. 18/dcn/09/18 -09 -0062 -00 -0000 -ofcom-srd-update. doc Submission 3 Andrew Gowans

May 2009 doc. : IEEE 802. 15 -09 -0435 -00 -0006 Contents • European Exempt Bands – Dedicated Medical bands in EC Decision – General use SRD bands that can be used • This years review of EC Decision – Proposed Medical Bands – Other medical band proposal Submission 4 Andrew Gowans

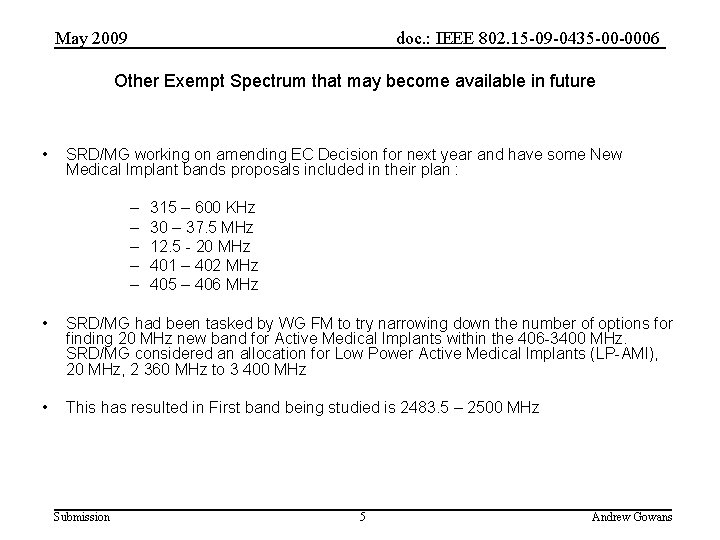
May 2009 doc. : IEEE 802. 15 -09 -0435 -00 -0006 Other Exempt Spectrum that may become available in future • SRD/MG working on amending EC Decision for next year and have some New Medical Implant bands proposals included in their plan : – – – 315 – 600 KHz 30 – 37. 5 MHz 12. 5 - 20 MHz 401 – 402 MHz 405 – 406 MHz • SRD/MG had been tasked by WG FM to try narrowing down the number of options for finding 20 MHz new band for Active Medical Implants within the 406 -3400 MHz. SRD/MG considered an allocation for Low Power Active Medical Implants (LP-AMI), 20 MHz, 2 360 MHz to 3 400 MHz • This has resulted in First band being studied is 2483. 5 – 2500 MHz Submission 5 Andrew Gowans

May 2009 doc. : IEEE 802. 15 -09 -0435 -00 -0006 andrew. gowans@ofcom. org. uk Submission 6 Andrew Gowans