Maximum Number of Electrons the formula 2 n

Maximum Number of Electrons • the formula 2 n 2 is used to find the max. # of e- on any energy level. - n = the energy level = (row on P. T. ) • Ex… • energy level 3… 2(3)2 = 18 e • energy level 6… 2(6)2 = 72 e-

Quantum numbers • Principal Quantum Numbers • Map to determine location of the electrons…. . • (Methods for denoting earrangement for an atom: orbital notation)

Quantum Numbers • the ‘address’ of an e-. • there are four quantum numbers (actually letters). . . 1 st = n = energy level of the e-. - ‘the principle quantum number’ - represented by rows on the periodic table - 1 = lowest, 7 = highest 2 nd = l = shape of sub-orbital - 4 different shapes…found on each energy level 3 rd = m = orientation of orbital in space. - which axis the orbital lies on 4 th = spin of the e- on its axis. - clockwise or counter-clockwise

1 st Quantum Number • 1. Principal Quantum Number (n) – Describes the size and energy of an orbital • ↑n ↑E ↑Distance from nucleus & ↓stability • Ex. n=1

nd 2 Quantum Number • l = shape of orbital • there are four orbital shapes, represented by letters… • each orbital can only hold 2 e-. • s = sphere = 1 orbital = 2 total e- lowest energy • p = dumbbell = 3 orbitals = 6 total e • d = clover-leaf = 5 orbitals = 10 total e • f = double clover-leaf = 7 orbitals = 14 total e- highest energy
2 nd Quantum number cont. • 2. Azmithual quantum number (l)describes the subshell of the orbital • l= n – 1 • if l = 0 then the electron is in the s subshell • • • l=1 l=2 l=3 p subshell d subshell f subshell
rd 3 Quantum number • 3. Magnetic (ml) – describes the 3 dimensional orientation of an orbital • Values of ml = -l to +l • Ex. if l = 0 then ml = just one orbital • If l = 1 then ml = 3 orbitals (-1, 0, 1 = x, y, z)
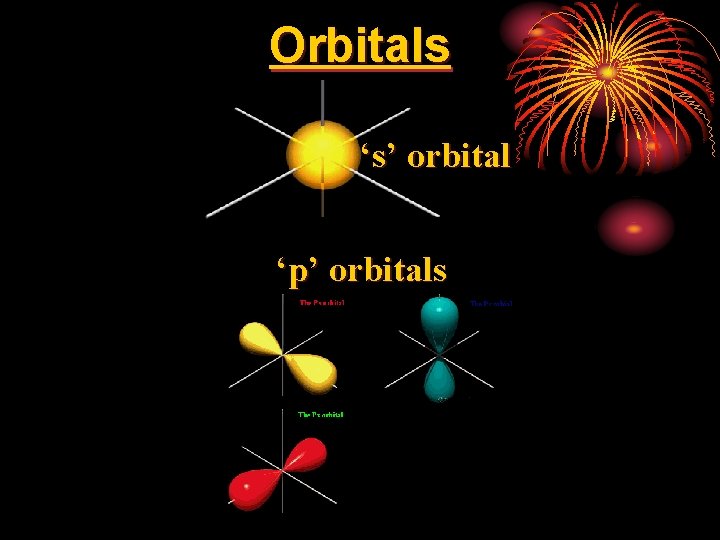
Orbitals ‘s’ orbital ‘p’ orbitals
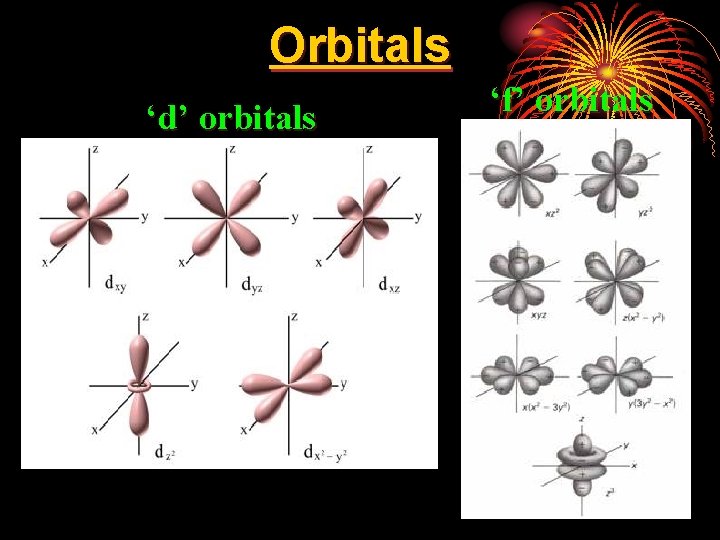
Orbitals ‘d’ orbitals ‘f’ orbitals

th 4 Quantum number • 4. Spin (ms) – describes the electron’s intrinsic magnetism • + ½ (spin up) or – ½ (spin down) • each e- occupies the lowest energy orbital available
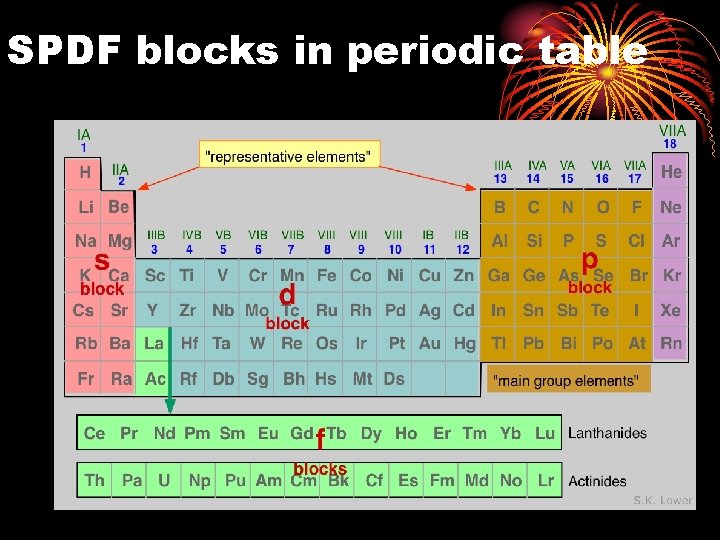
SPDF blocks in periodic table •

Quantum Numbers Practice • What are the 4 quantum numbers of: • The • The last electron in P 5 th electron in Na 9 th electron in Cl 5 th electron in O last electron in Cu last electron in Fe
- Slides: 12