MATTER yes Can it be physically separated MIXTURE
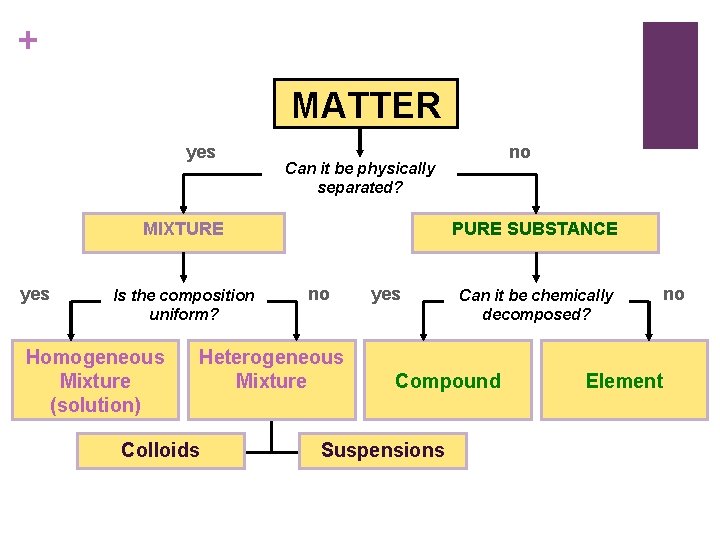
+ MATTER yes Can it be physically separated? MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no yes Can it be chemically decomposed? Compound Suspensions no Element
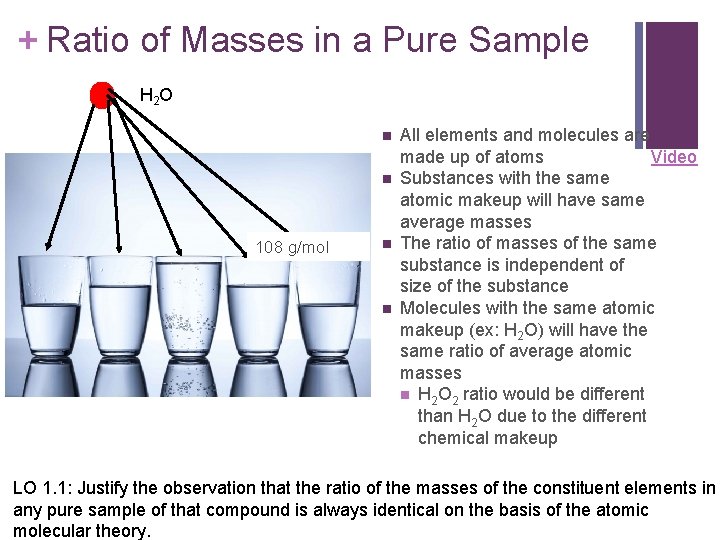
+ Ratio of Masses in a Pure Sample H 2 O n n 108 g/mol n n All elements and molecules are made up of atoms Video Substances with the same atomic makeup will have same average masses The ratio of masses of the same substance is independent of size of the substance Molecules with the same atomic makeup (ex: H 2 O) will have the same ratio of average atomic masses n H 2 O 2 ratio would be different than H 2 O due to the different chemical makeup LO 1. 1: Justify the observation that the ratio of the masses of the constituent elements in any pure sample of that compound is always identical on the basis of the atomic molecular theory.
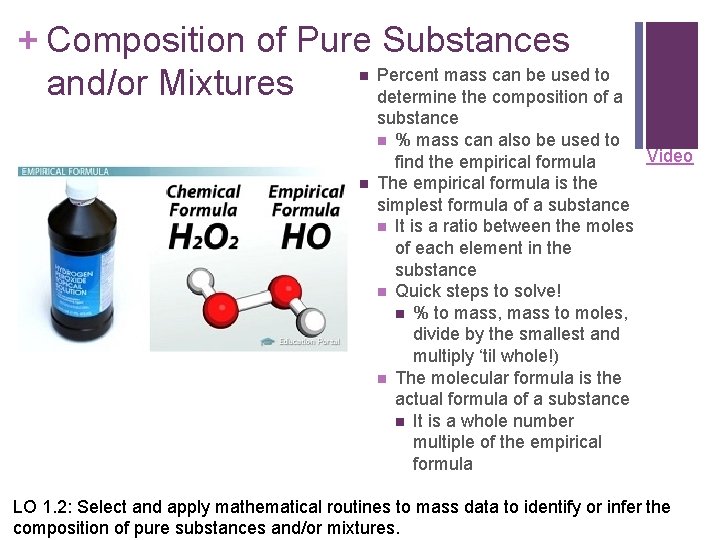
+ Composition of Pure Substances Percent mass can be used to and/or Mixtures determine the composition of a n n substance n % mass can also be used to Video find the empirical formula The empirical formula is the simplest formula of a substance n It is a ratio between the moles of each element in the substance n Quick steps to solve! n % to mass, mass to moles, divide by the smallest and multiply ‘til whole!) n The molecular formula is the actual formula of a substance n It is a whole number multiple of the empirical formula LO 1. 2: Select and apply mathematical routines to mass data to identify or infer the composition of pure substances and/or mixtures.
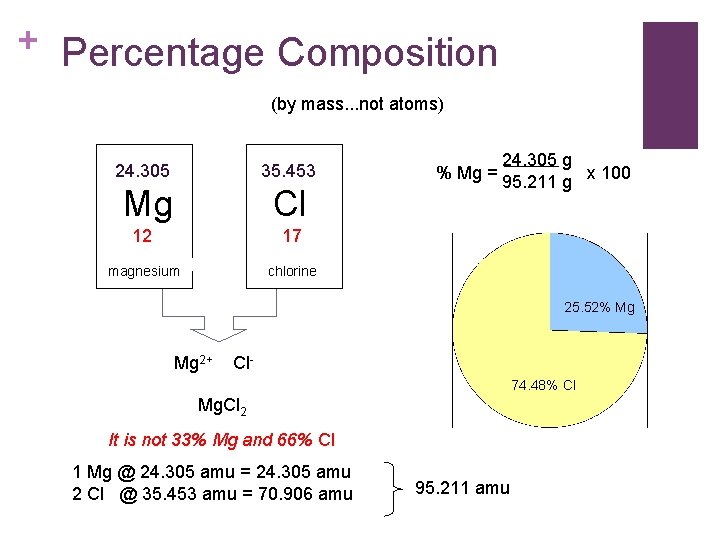
+ Percentage Composition (by mass. . . not atoms) 24. 305 35. 453 Mg Cl 12 17 magnesium chlorine 24. 305 g % Mg = 95. 211 g x 100 25. 52% Mg Mg 2+ Cl 74. 48% Cl Mg. Cl 2 It is not 33% Mg and 66% Cl 1 Mg @ 24. 305 amu = 24. 305 amu 2 Cl @ 35. 453 amu = 70. 906 amu 95. 211 amu
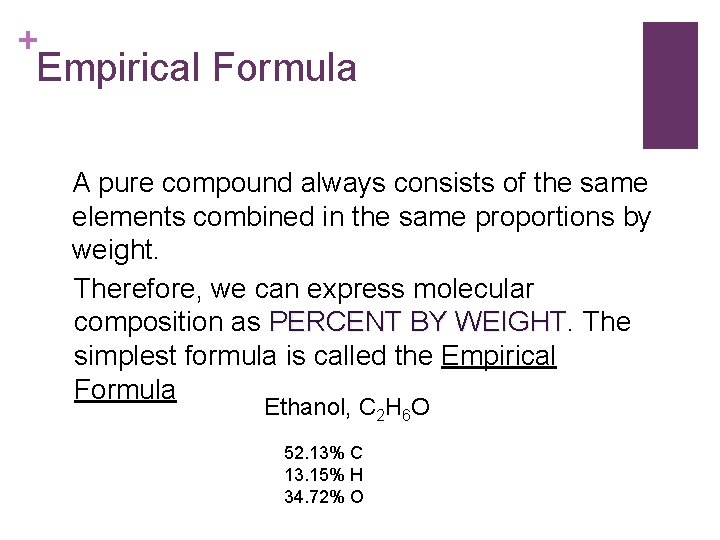
+ Empirical Formula A pure compound always consists of the same elements combined in the same proportions by weight. Therefore, we can express molecular composition as PERCENT BY WEIGHT The simplest formula is called the Empirical Formula Ethanol, C 2 H 6 O 52. 13% C 13. 15% H 34. 72% O
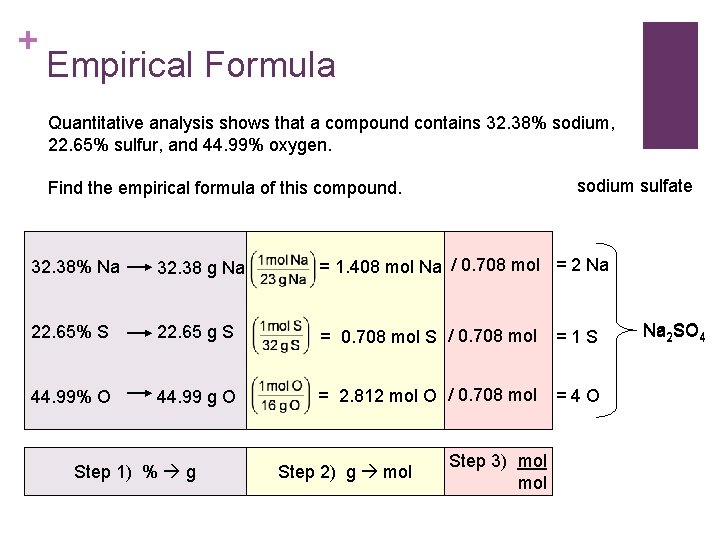
+ Empirical Formula Quantitative analysis shows that a compound contains 32. 38% sodium, 22. 65% sulfur, and 44. 99% oxygen. sodium sulfate Find the empirical formula of this compound. 32. 38% Na 32. 38 g Na = 1. 408 mol Na / 0. 708 mol = 2 Na 22. 65% S 22. 65 g S = 0. 708 mol S / 0. 708 mol =1 S 44. 99% O 44. 99 g O = 2. 812 mol O / 0. 708 mol =4 O Step 1) % g Step 2) g mol Step 3) mol Na 2 SO 4
+ Identifying Purity of a Substance n n n Impurities in a substance can change the percent composition by mass Video If more of a certain element is added from an impurity, then the percent mass of that element will increase and vice versa When heating a hydrate, the substance is heated several times to ensure the water is driven off n Then you are simply left with the pure substance and no excess water LO 1. 3: The student is able to select and apply mathematical relationships to mass data in order to justify a claim regarding the identity and/or estimated purity of a substance.
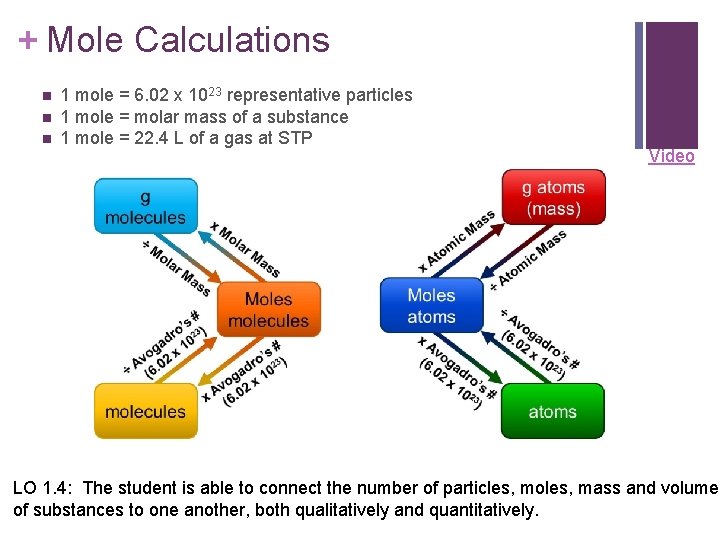
+ Mole Calculations n n n 1 mole = 6. 02 x 1023 representative particles 1 mole = molar mass of a substance 1 mole = 22. 4 L of a gas at STP Video LO 1. 4: The student is able to connect the number of particles, moles, mass and volume of substances to one another, both qualitatively and quantitatively.
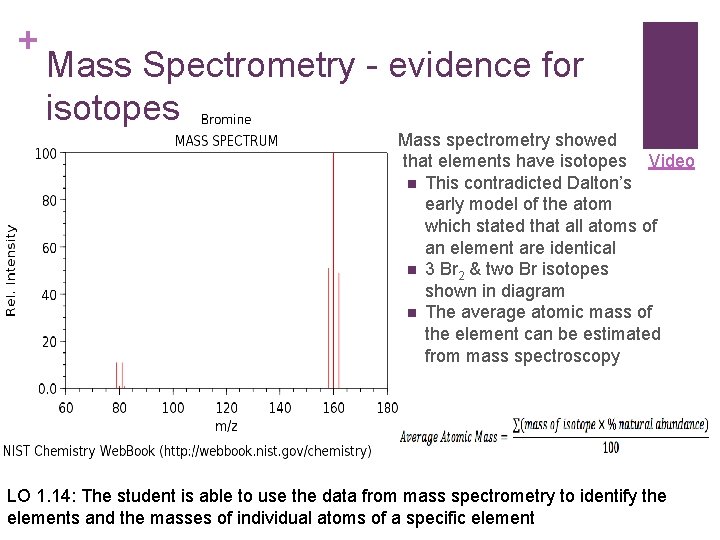
+ Mass Spectrometry - evidence for isotopes Mass spectrometry showed that elements have isotopes Video n This contradicted Dalton’s early model of the atom which stated that all atoms of an element are identical n 3 Br 2 & two Br isotopes shown in diagram n The average atomic mass of the element can be estimated from mass spectroscopy LO 1. 14: The student is able to use the data from mass spectrometry to identify the elements and the masses of individual atoms of a specific element
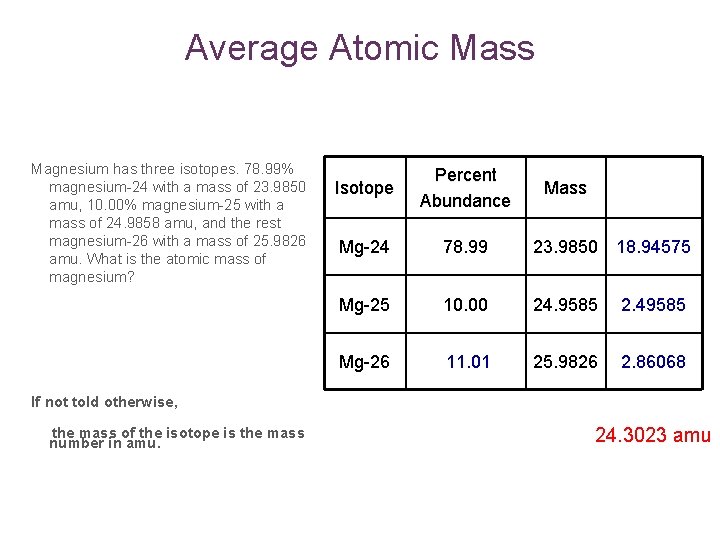
Average Atomic Mass Magnesium has three isotopes. 78. 99% magnesium-24 with a mass of 23. 9850 amu, 10. 00% magnesium-25 with a mass of 24. 9858 amu, and the rest magnesium-26 with a mass of 25. 9826 amu. What is the atomic mass of magnesium? Isotope Percent Abundance Mass Mg-24 78. 99 23. 9850 18. 94575 Mg-25 10. 00 24. 9585 2. 49585 Mg-26 11. 01 25. 9826 2. 86068 If not told otherwise, the mass of the isotope is the mass number in amu. 24. 3023 amu
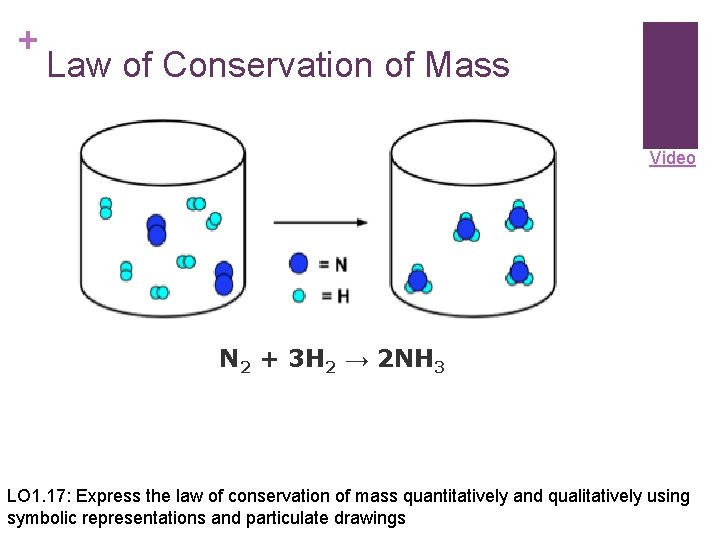
+ Law of Conservation of Mass Video N 2 + 3 H 2 → 2 NH 3 LO 1. 17: Express the law of conservation of mass quantitatively and qualitatively using symbolic representations and particulate drawings
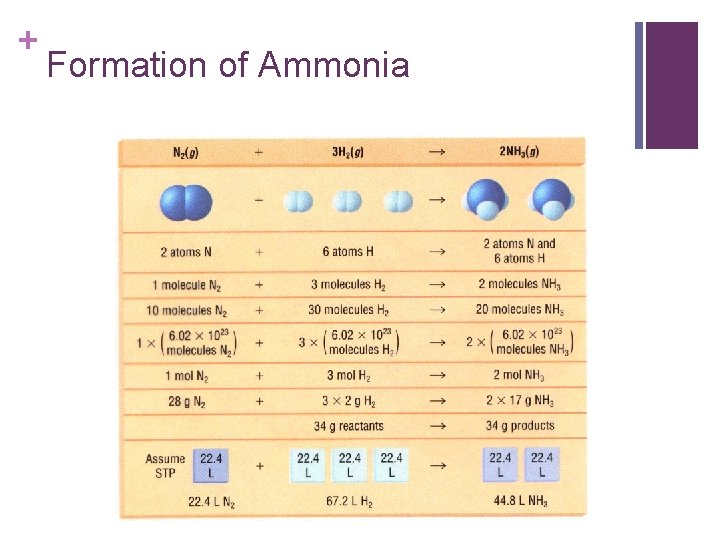
+ Formation of Ammonia
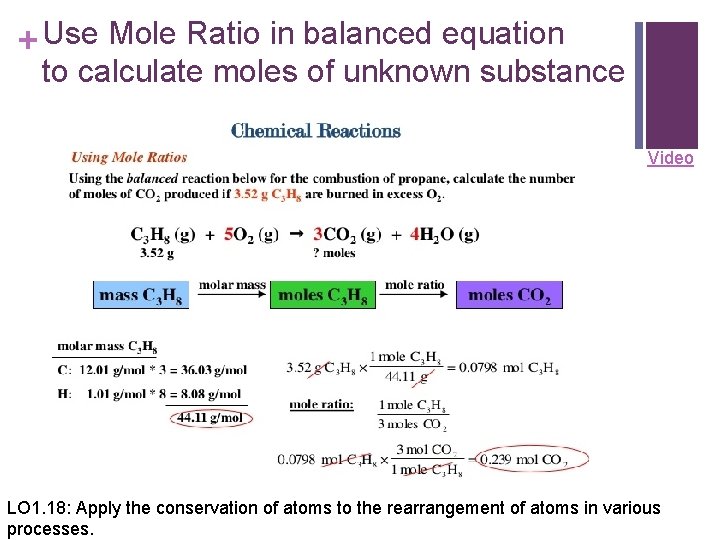
+ Use Mole Ratio in balanced equation to calculate moles of unknown substance Video LO 1. 18: Apply the conservation of atoms to the rearrangement of atoms in various processes.
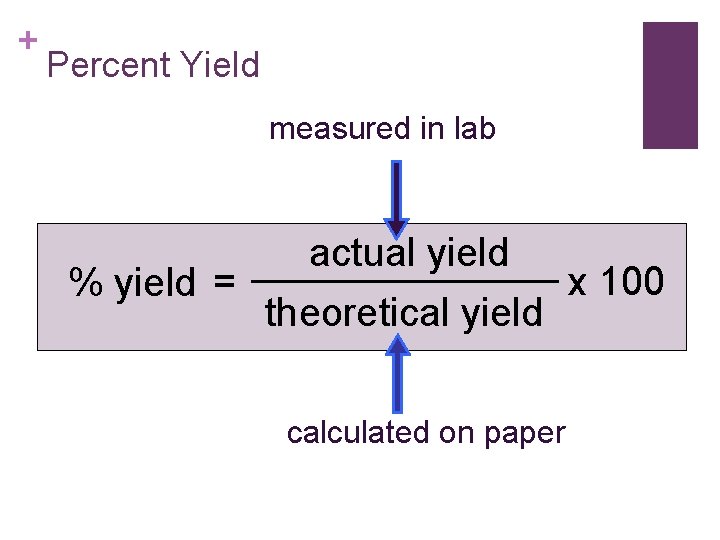
+ Percent Yield measured in lab % yield = actual yield theoretical yield calculated on paper x 100
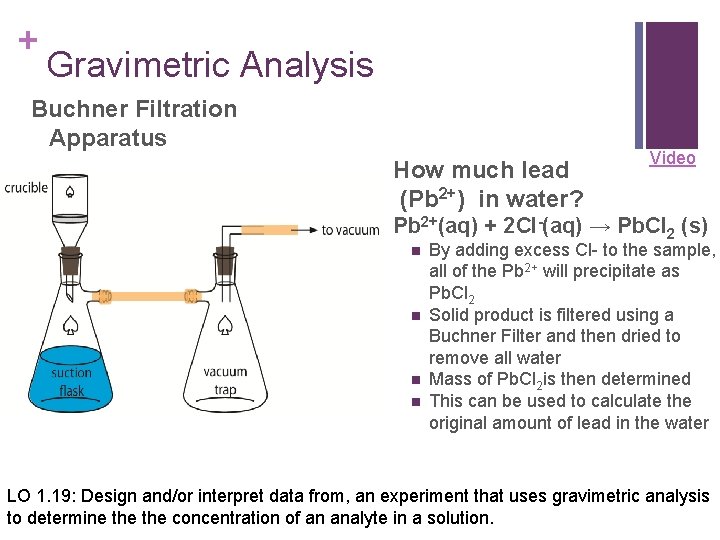
+ Gravimetric Analysis Buchner Filtration Apparatus How much lead (Pb 2+) in water? Video Pb 2+(aq) + 2 Cl-(aq) → Pb. Cl 2 (s) n n By adding excess Cl- to the sample, all of the Pb 2+ will precipitate as Pb. Cl 2 Solid product is filtered using a Buchner Filter and then dried to remove all water Mass of Pb. Cl 2 is then determined This can be used to calculate the original amount of lead in the water LO 1. 19: Design and/or interpret data from, an experiment that uses gravimetric analysis to determine the concentration of an analyte in a solution.
- Slides: 15