MATTER WHAT IS MATTER Anything that has mass











- Slides: 23

MATTER

WHAT IS MATTER? Anything that has mass and takes up space.

STATES OF MATTER • Solids- tightly packed molecules, definite shape, does not conform to shape of container. • Liquids- “slippery” molecules, definite volume, conforms to shape of container. • Gases- lots of space between molecules, takes up all the volume of a container.

ELEMENTS • Found on the Periodic Table • Cannot be separated into smaller pieces and still retain the characteristics of the element.

COMPOUNDS • Made of 2 or more elements chemically combined together. • Have a FORMULA!

MIXTURES • Two or more substances mixed together. • NO chemical reaction has taken place! • Can be separated by physical means.

HOMOGENEOUS MIXTURE • Uniform in appearance • No visible “chunks” • Metal mixtures are alloys.

HETEROGENEOUS MIXTURES • Not uniform in appearance • Has visible “chunks”

Classify the following by element, compound or mixture. If the substance is a mixture, identify whether it is homogeneous or heterogeneous. 1. Na. Cl 2. Kool-Aid 3. Potting soil 4. 14 K gold 5. Vegetable soup 6. Ca. CO 3 7. Magnesium sulfate 8. Aluminum 9. Hydrogen 10. Creamy peanut butter

Basic Atomic Structure

EARLY THEORIES Democritus’s 460 – 370 B. C. • Matter is composed of empty space through which atoms move. • Atoms are solid, homogeneous, indestructible, and indivisible. • Different kinds of atoms have different sizes and shapes. • The differing properties of matter are due to the size, shape, and movement of atoms. • Apparent changes in matter result from changes in the groupings of atoms and not from changes in the atoms themselves.

Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter reemerged in the early 19 th century, championed by John Dalton.

Dalton’s Postulates • • Each element is composed of extremely small particles called atoms. All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements. Atoms of an element are not changed into atoms of a different element by chemical reactions; atoms are neither created nor destroyed in chemical reactions. Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms.

The Electron • Streams of negatively charged particles were found to emanate from cathode tubes. • J. J. Thompson is credited with their discovery (1897).

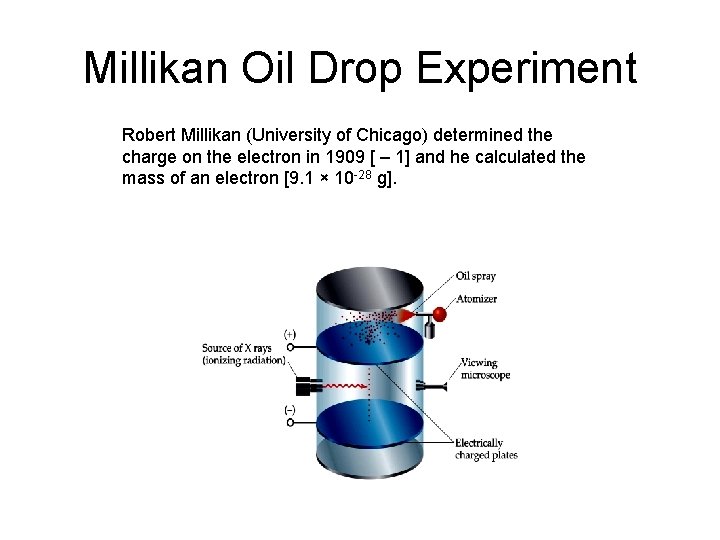
Millikan Oil Drop Experiment Robert Millikan (University of Chicago) determined the charge on the electron in 1909 [ – 1] and he calculated the mass of an electron [9. 1 × 10 -28 g].

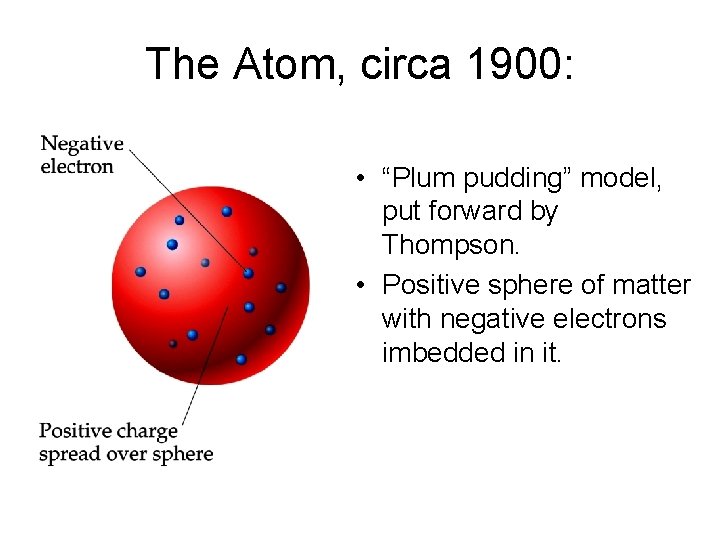
The Atom, circa 1900: • “Plum pudding” model, put forward by Thompson. • Positive sphere of matter with negative electrons imbedded in it.

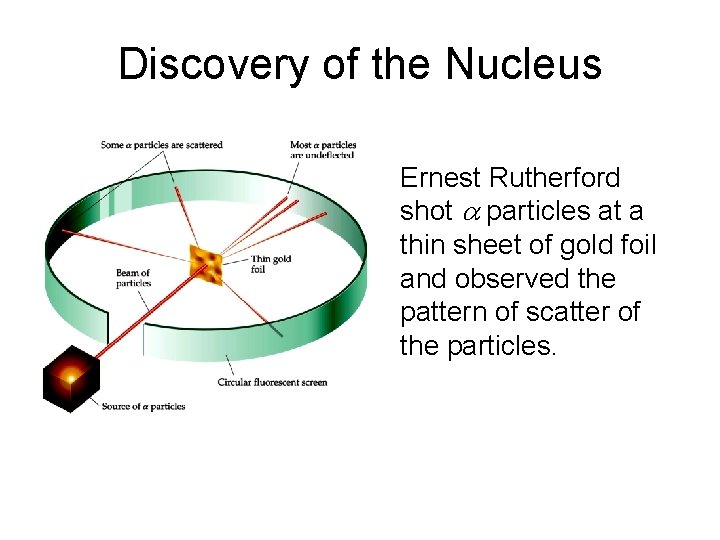
Discovery of the Nucleus Ernest Rutherford shot particles at a thin sheet of gold foil and observed the pattern of scatter of the particles.

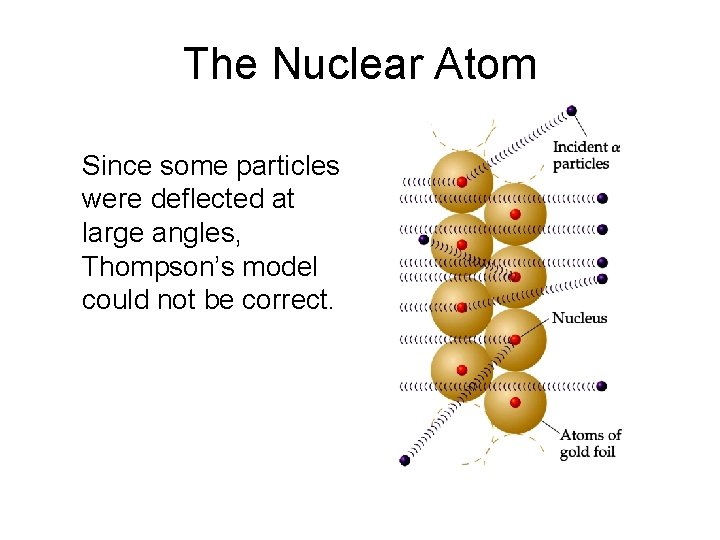
The Nuclear Atom Since some particles were deflected at large angles, Thompson’s model could not be correct.

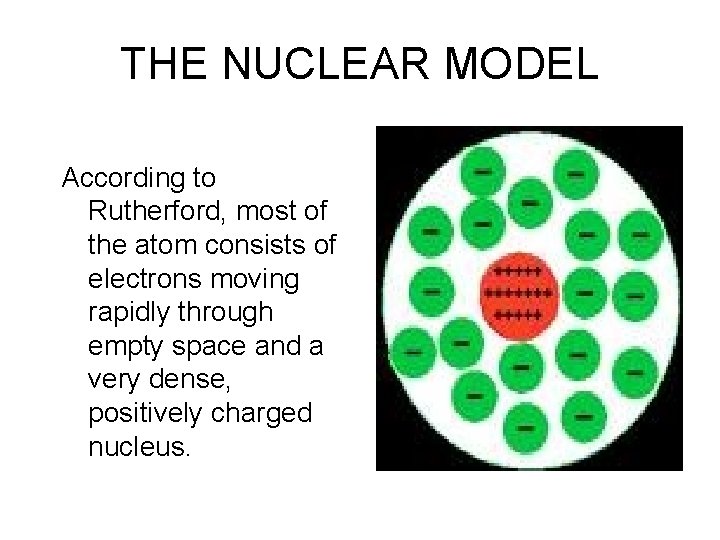
THE NUCLEAR MODEL According to Rutherford, most of the atom consists of electrons moving rapidly through empty space and a very dense, positively charged nucleus.

Other Subatomic Particles • By 1920 , Rutherford had refined his concept of the nucleus: He concluded that the very dense nucleus contained positively particles called protons. • James Chadwick, a coworker, showed that the nucleus also contained a neutral particle in 1932. This was the neutron – a particle with nearly equal mass as a proton.

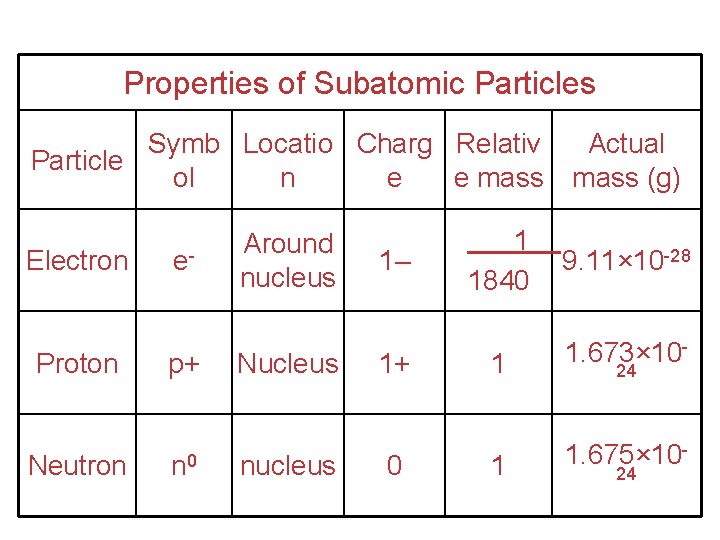
Properties of Subatomic Particles Symb Locatio Charg Relativ Particle ol n e e mass Electron e- Proton p+ Neutron n 0 Around nucleus Nucleus nucleus 1– 1+ 0 Actual mass (g) 1 1840 9. 11× 10 -28 1 1. 673× 10 - 1 1. 675× 10 - 24 24

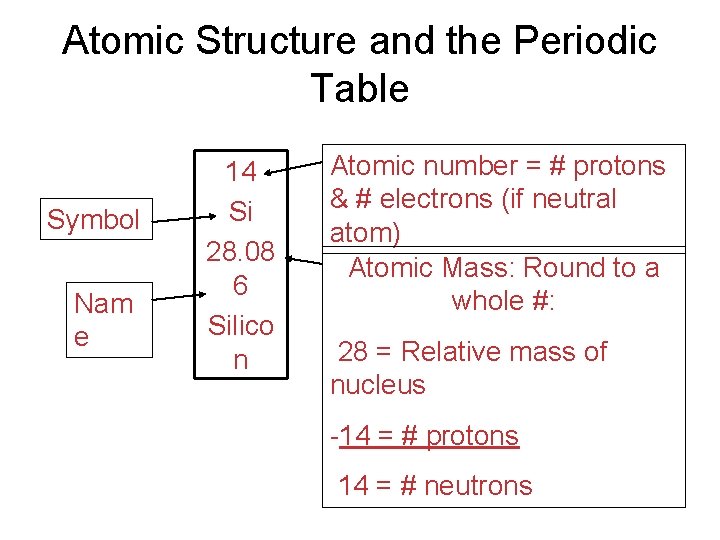
Atomic Structure and the Periodic Table Symbol Nam e 14 Si 28. 08 6 Silico n Atomic number = # protons & # electrons (if neutral atom) Atomic Mass: Round to a whole #: 28 = Relative mass of nucleus -14 = # protons 14 = # neutrons

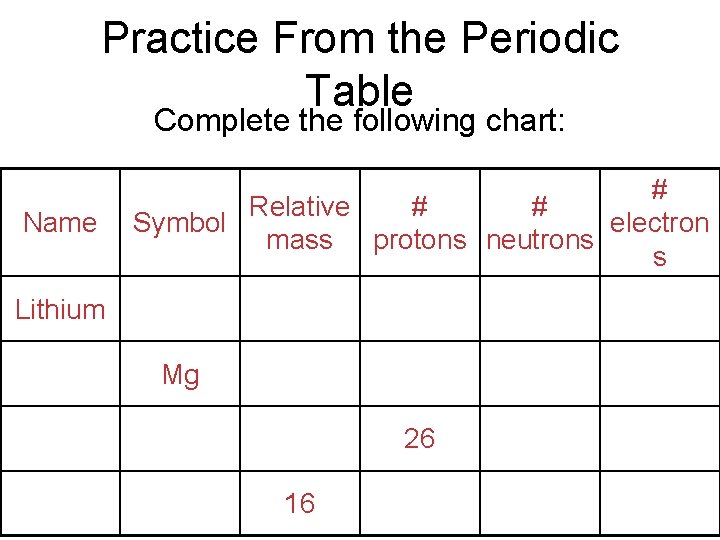
Practice From the Periodic Table Complete the following chart: Name # Relative # # electron Symbol mass protons neutrons s Lithium Mg 26 16
 That which occupies space and has mass
That which occupies space and has mass Compare and contrast template
Compare and contrast template All matter has and takes up
All matter has and takes up What is mass
What is mass Matter is anything that occupies
Matter is anything that occupies It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter has mass and occupies space
Matter has mass and occupies space Matter is anything that has
Matter is anything that has Is anything that has mass and volume
Is anything that has mass and volume Is anything that has mass and takes up space.
Is anything that has mass and takes up space. No matter anything
No matter anything Matter is anything that has both
Matter is anything that has both Anthing that takes up space and has mass is called?
Anthing that takes up space and has mass is called? Anything that has mass and takes up space
Anything that has mass and takes up space Something that takes up space
Something that takes up space Matter is anything that occupies space
Matter is anything that occupies space Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block nhĩ thất độ 2 type 1
Block nhĩ thất độ 2 type 1 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai








































