Matter What is It Anything that has mass












- Slides: 23

Matter

What is It? • Anything that has mass and takes up space. • Exists in two chemical forms: – Elements: Composed of one type of atom • Oxygen • Carbon – Compounds: Composed of two or more elements held together by bonds • Water • Salt

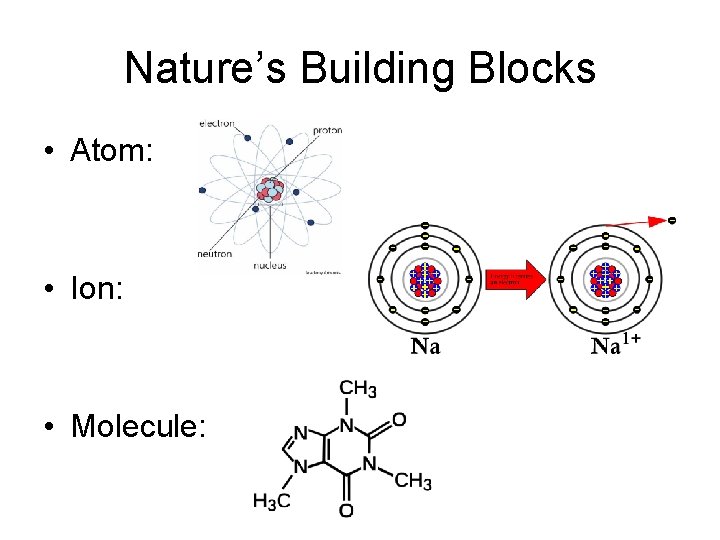
Nature’s Building Blocks • Atom: • Ion: • Molecule:

Diversity of Life • The molecules that comprise organisms on Earth are made of chemicals that are based on the Carbon atom (Organic Chemistry). • Carbon is very versatile and can form various macromolecules. • “Carbon Based Life Forms”

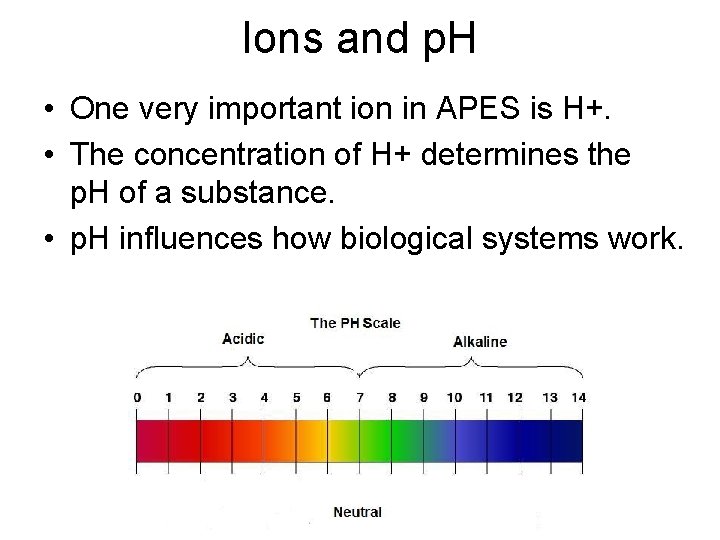
Ions and p. H • One very important ion in APES is H+. • The concentration of H+ determines the p. H of a substance. • p. H influences how biological systems work.

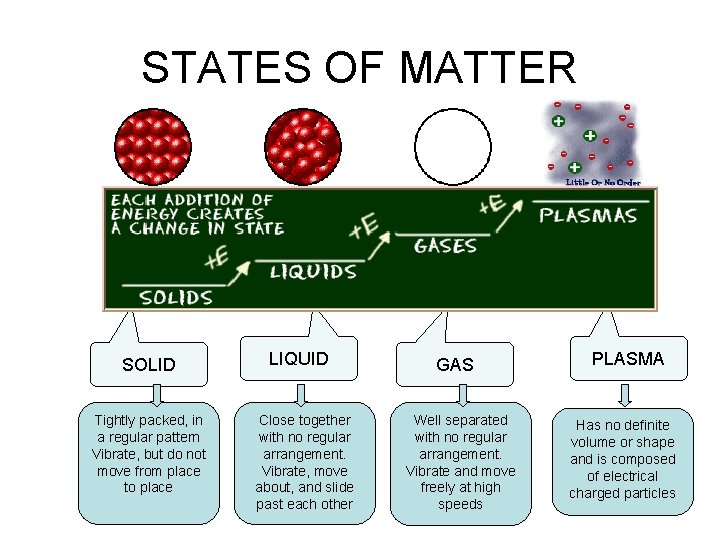
STATES OF MATTER • The Four States of Matter • Solid • Liquid • Gas • Plasma

STATES OF MATTER Ø Based upon particle arrangement Ø Based upon energy of particles Ø Based upon distance between particles

Kinetic Theory of Matter is made up of particles which are in continual random motion.

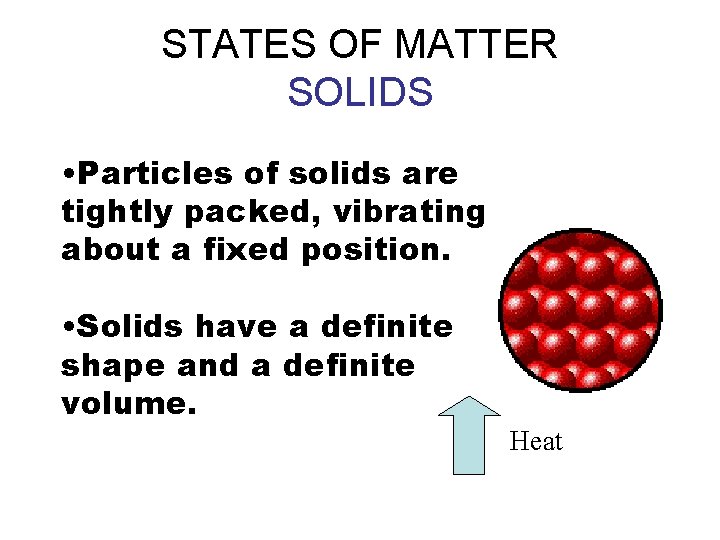
STATES OF MATTER SOLIDS • Particles of solids are tightly packed, vibrating about a fixed position. • Solids have a definite shape and a definite volume. Heat

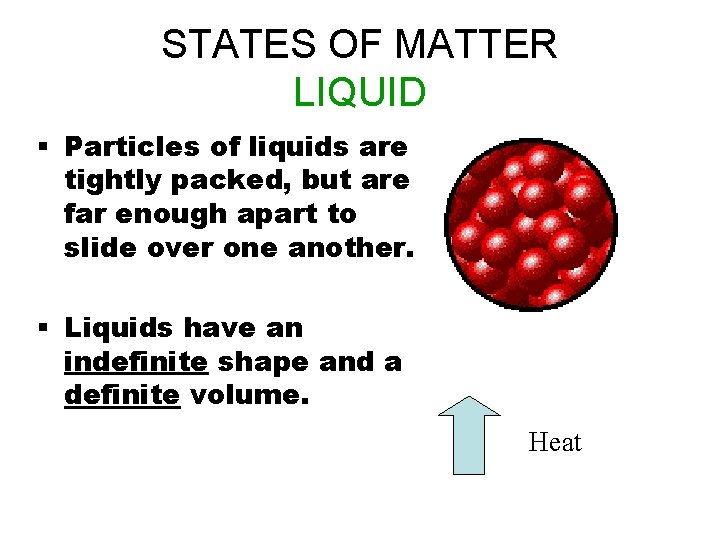
STATES OF MATTER LIQUID § Particles of liquids are tightly packed, but are far enough apart to slide over one another. § Liquids have an indefinite shape and a definite volume. Heat

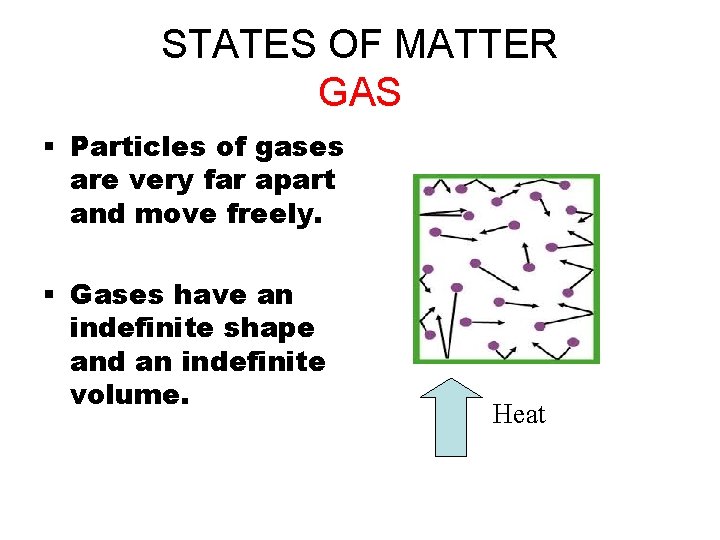
STATES OF MATTER GAS § Particles of gases are very far apart and move freely. § Gases have an indefinite shape and an indefinite volume. Heat

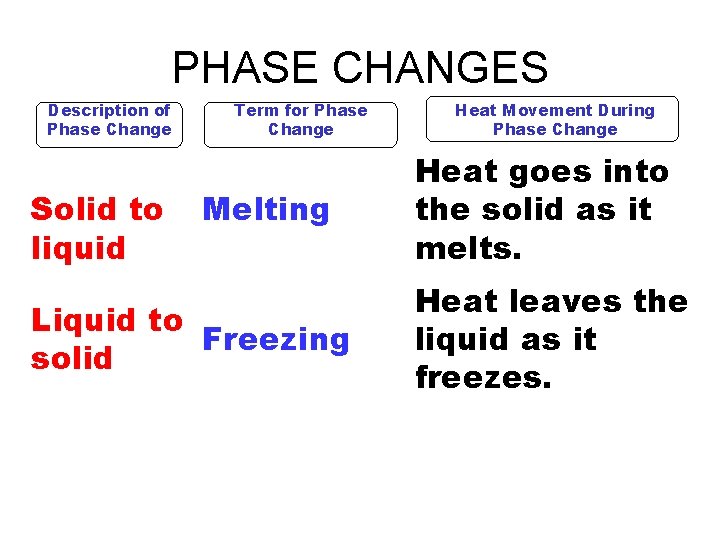
PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

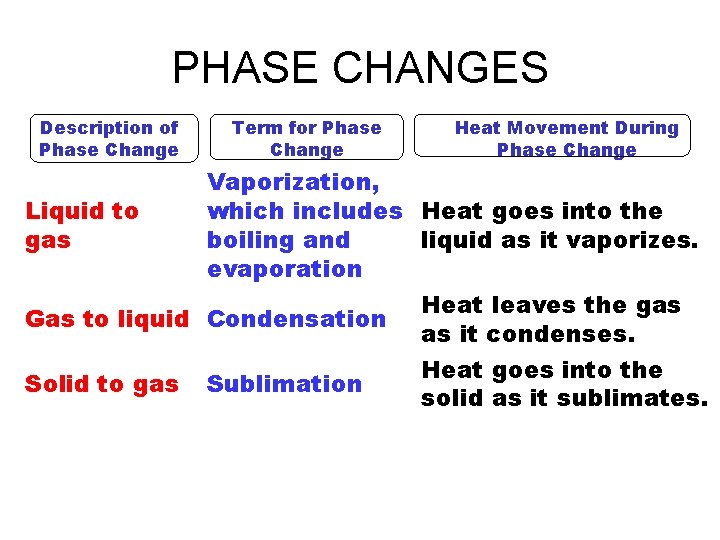
PHASE CHANGES Description of Phase Change Term for Phase Change Heat Movement During Phase Change Vaporization, which includes Heat goes into the Liquid to gas boiling and liquid as it vaporizes. evaporation Heat leaves the gas Gas to liquid Condensation as it condenses. Heat goes into the Solid to gas Sublimation solid as it sublimates.

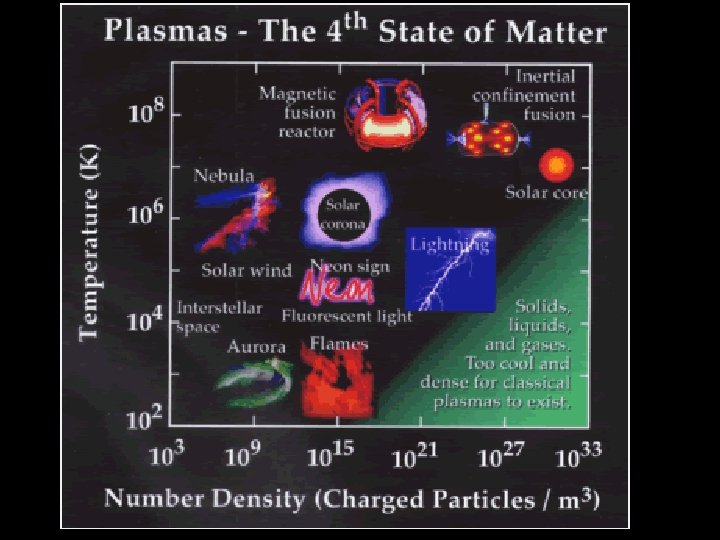
But what happens if you raise the temperature to super-high levels… between 1000°C and 1, 000, 000°C ? Will everything just be a gas?

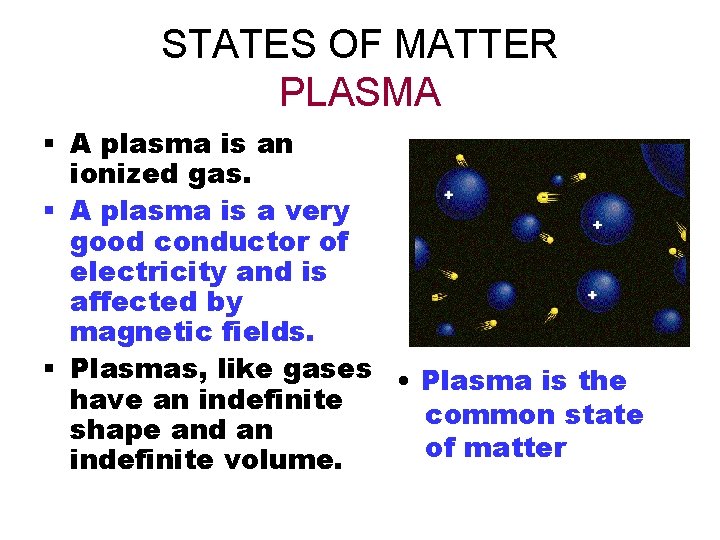
STATES OF MATTER PLASMA § A plasma is an ionized gas. § A plasma is a very good conductor of electricity and is affected by magnetic fields. § Plasmas, like gases • Plasma is the have an indefinite common state shape and an of matter indefinite volume.

STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

Some places where plasmas are found… 1. Flames

2. Lightning

3. Aurora (Northern Lights)

The Sun is an example of a star in its plasma state


Matter Quality • Classified as high quality or low quality depending on how useful it is to us as a resource. – High quality: concentrated, found near earth’s surface, has great potential use as a matter resource – Low quality: dilute, often deep underground or dispersed in the ocean or atmosphere, has little potential for material resource VS

Material Efficiency • AKA resource productivity – Total amount of material needed to produce each unit of goods or services