MATTER TABLE OF CONTENTS 2 1 Classification of

MATTER

TABLE OF CONTENTS • 2. 1 Classification of Matter Slides 3 - 9 • 2. 2 Concepts of Matter Slides 11 - 22 • 2. 3 States of Matter Slides 24 - 31

CLASSIFICATION OF MATTER C. 4. D classify matter as pure substances or mixtures through investigation of their properties.

MATTER: • Matter is defined as anything that has mass and takes up space • Chemists use characteristic properties to tell substances apart and to separate them • Matter can be classified as either a substance or a mixture. Substance is matter that has a uniform and definite composition Mixture is matter that does not have a uniform composition
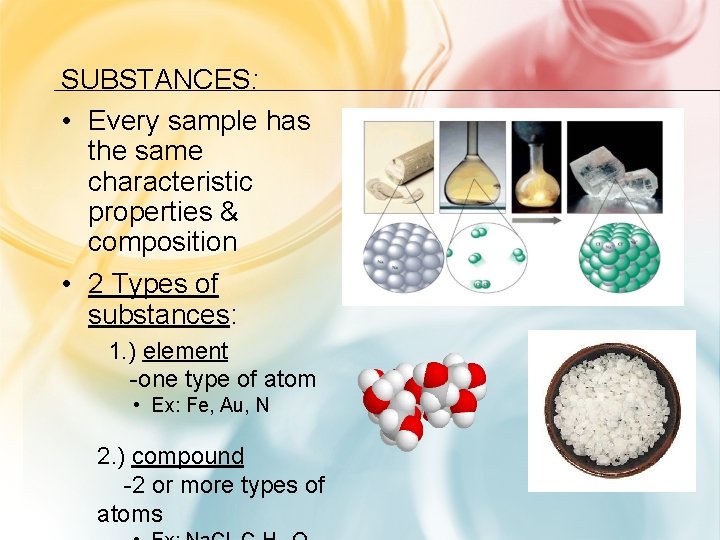
SUBSTANCES: • Every sample has the same characteristic properties & composition • 2 Types of substances: 1. ) element -one type of atom • Ex: Fe, Au, N 2. ) compound -2 or more types of atoms

MIXTURES: • Blend of 2 or more types of matter • Each component keeps its own identity and properties • The components are only physically mixed • Can be separated using physical means • Mixtures are classified as either homogeneous or heterogeneous

HOMOGENEOUS MIXTURES (SOLUTIONS) • Uniform in composition Ex: • No visible parts • air • Also called solution • salt water • 2 parts of a solution 1. ) solvent –does • brass the dissolving 2. ) solute- gets dissolved • vinegar

HETEROGENEOUS MIXTURES • Not uniform in composition • Visible parts Ex: • soil • concrete • blood • chocolate chip cookies • sand in water • iced tea with ice
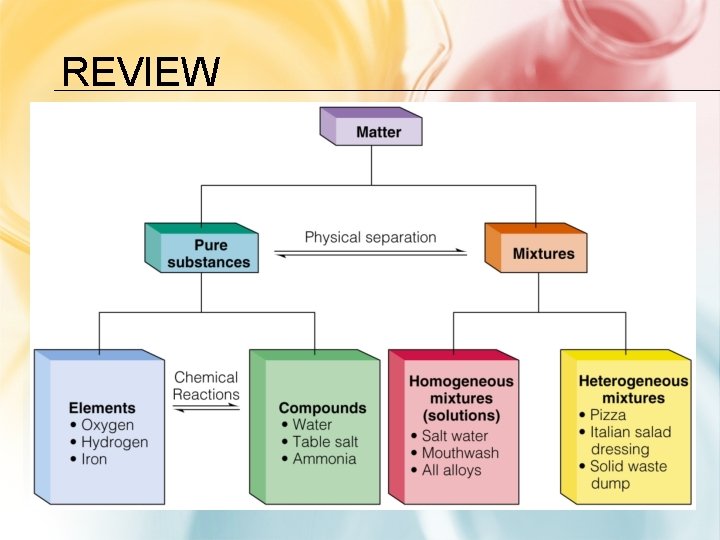
REVIEW

FOLLOWING IS ELEMENT, COMPOUND, HOMOGENEOUS MIXTURE OR HETEROGENEOUS MIXTURE. • • • air Zinc (Zn) Chlorine (Cl) granite aluminum (Al) sugar in water blood Sucrose (C 12 H 22 O 11) stainless steel sodium chloride (Na. Cl) • • • brass whole milk apple table salt (Na. Cl) soft drinks vinegar concrete sodium (Na) baking soda (Na. HCO 3) gravel

CONCEPTS OF MATTER C. 4. A differentiate between physical and chemical changes and properties

PHYSICAL PROPERTIES • Any quality or condition of a substance that can be observed or measured without changing the substances identity. • Physical properties can be classified as Intensive and Extensive properties.

INTENSIVE AND EXTENSIVE PHYSICAL PROPERTIES • Intensive Property is a physical property that does not depend on the system size or the amount of material in the system. • Examples of intensive properties include: * temperature * viscosity * density * melting point * boiling point • Extensive Property is a physical property that does depend on the system size or the amount of material in the system. • Examples of extensive properties include: * mass * volume * length

CHEMICAL PROPERTIES • Chemical Property is how a substance reacts in the presence of: • Air • Acids • Water • Bases • Chemicals • Heat
PHYSICAL CHANGES IN MATTER • Change in a substance that doesn’t change the identity of the substance • Includes all changes of state (solid, liquid, or gas) Ex. grinding, cutting, melting, boiling
CHEMICAL CHANGES IN MATTER • Chemical Change is a change in which a substance is converted into a different substance • Doesn’t change the amount of matter present • reactants – substances that react (left side of the equation) • products – substances that form (right side of the equation)
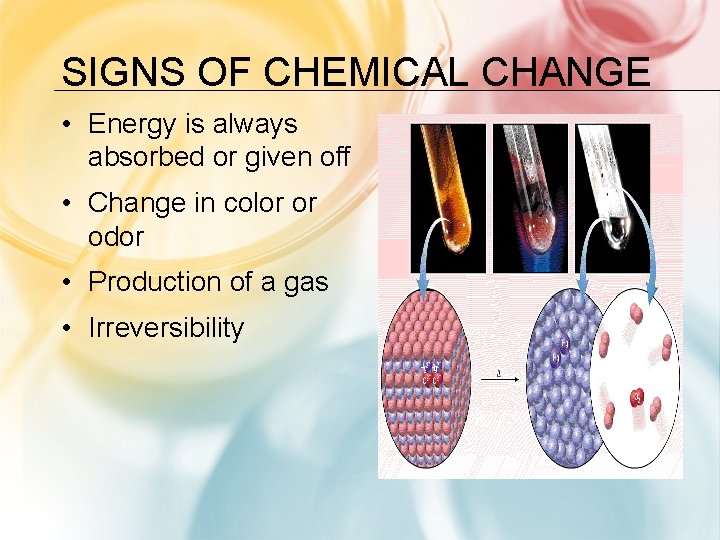
SIGNS OF CHEMICAL CHANGE • Energy is always absorbed or given off • Change in color or odor • Production of a gas • Irreversibility
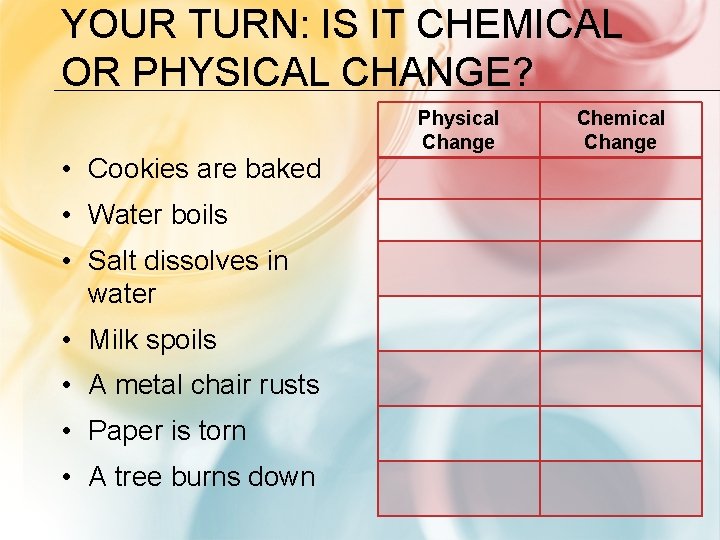
YOUR TURN: IS IT CHEMICAL OR PHYSICAL CHANGE? • Cookies are baked • Water boils • Salt dissolves in water • Milk spoils • A metal chair rusts • Paper is torn • A tree burns down Physical Change Chemical Change


MIXTURE SEPARATION TECHNIQUES • Filtration- solid part is trapped by filter paper and the liquid part runs through the paper • Vaporization- where the liquid portion is evaporated off to leave solid
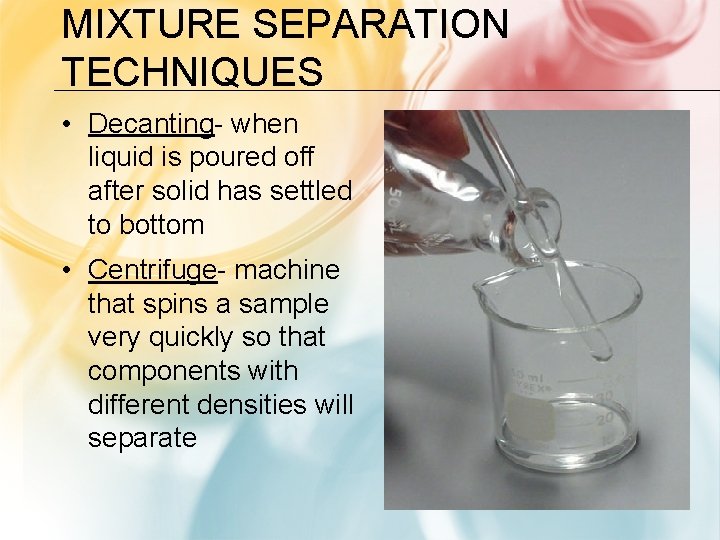
MIXTURE SEPARATION TECHNIQUES • Decanting- when liquid is poured off after solid has settled to bottom • Centrifuge- machine that spins a sample very quickly so that components with different densities will separate

MIXTURE SEPARATION TECHNIQUES • Paper Chromatography- used to separate mixtures because different parts move quicker on paper than other


STATES OF MATTER C. 4. C Compare solids, liquids, and gases in terms of compressibility, structure, Shape and volume.

PHASES OF MATTER: • Solid • Liquid • Gas
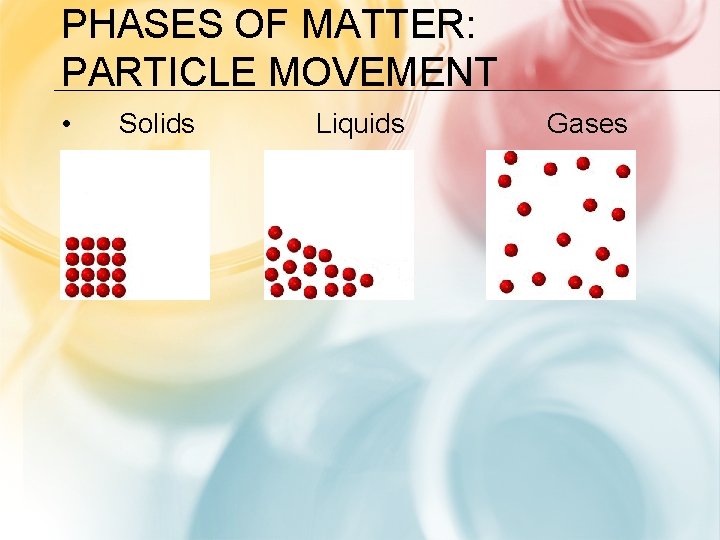
PHASES OF MATTER: PARTICLE MOVEMENT • Solids Liquids Gases
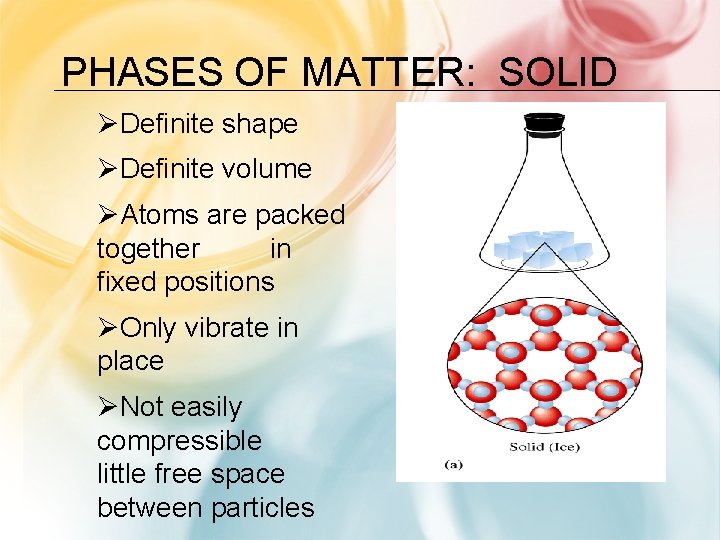
PHASES OF MATTER: SOLID ØDefinite shape ØDefinite volume ØAtoms are packed together in fixed positions ØOnly vibrate in place ØNot easily compressible little free space between particles
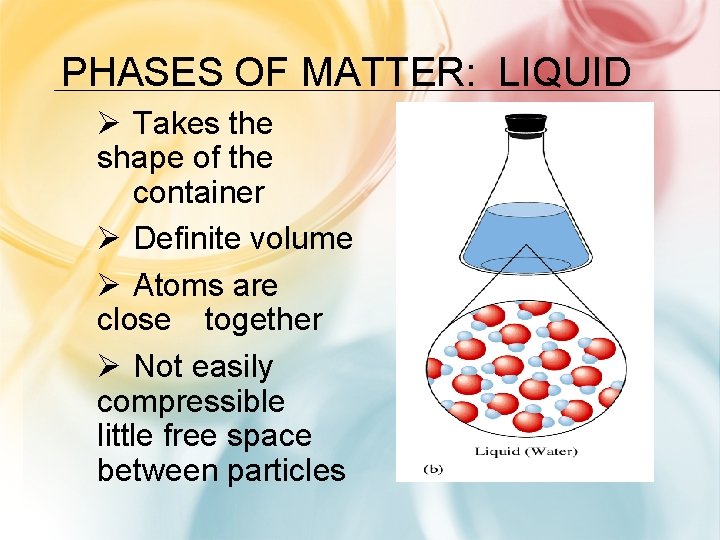
PHASES OF MATTER: LIQUID Ø Takes the shape of the container Ø Definite volume Ø Atoms are close together Ø Not easily compressible little free space between particles
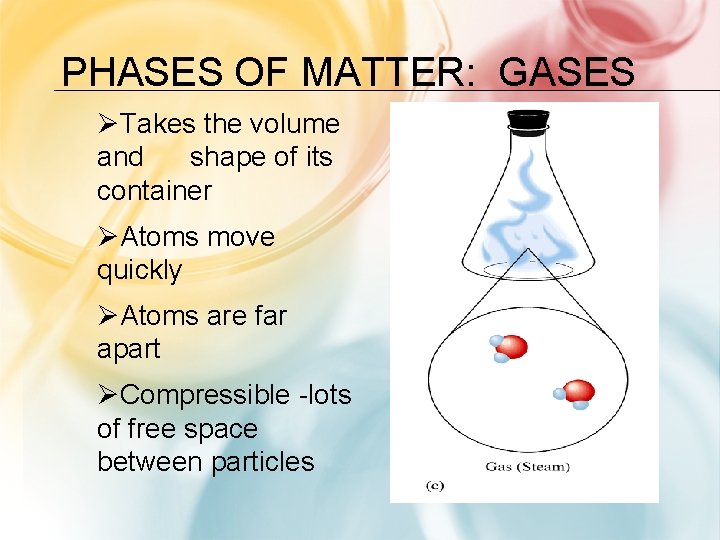
PHASES OF MATTER: GASES ØTakes the volume and shape of its container ØAtoms move quickly ØAtoms are far apart ØCompressible -lots of free space between particles
CHEMICAL REACTIONS -One or more substances change into new substances. 2 Na + Cl 2 ------ 2 Na. Cl Reactants -Starting substances -Written to the left of the arrow Products -Substances formed -Written to the right of the arrow Arrow -”produce”, “yield”, “change into”
LAW OF CONSERVATION OF MASS -Mass can be neither created nor destroyed in an ordinary chemical or physical process ** mass of the reactants = mass of the products** Ex: How many grams of Na. Cl are produced if 12. 5 g of Na and 25. 7 g of Cl 2 reacted?

- Slides: 32