Matter Subatomic Particles Bonding 1 What is matter

Matter, Subatomic Particles & Bonding

1. What is matter? • Anything that occupies space and has mass • Has to have both!!!

2. What is an element? • Substances that can not be broken down into other substances

3. What is an atom? • Smallest amount of an element by physically making it smaller

4. This is picture of a? • Water molecule • 5 A. There are ____ hydrogen (H) atoms. • 5 B. There are _____ (O) oxygen atoms. • 6. How do you write the formula? • H 2 O

7. In the formula, where does the # of elements go? • After the letter as a subscript • H 2 0 • Why isn’t there a number after the O? • Is water an element or compound or molecule? • How do you know?

8. How are the oxygen and hydrogen held together? • bonds • All compounds are based upon different types of bonds!
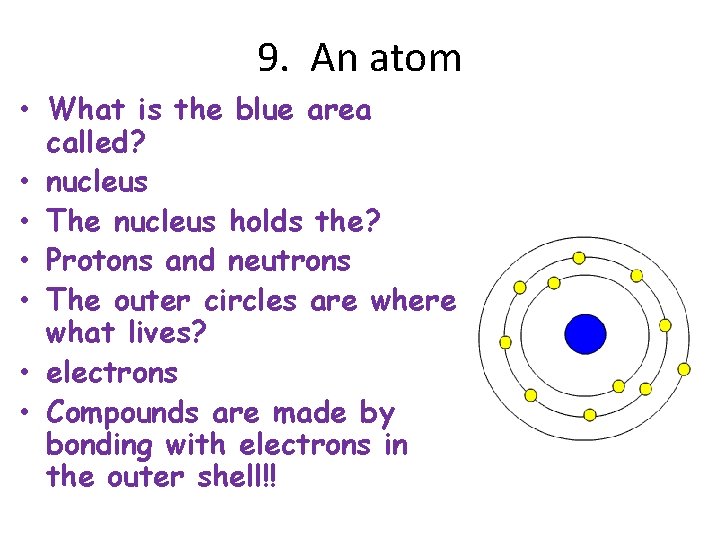
9. An atom • What is the blue area called? • nucleus • The nucleus holds the? • Protons and neutrons • The outer circles are where what lives? • electrons • Compounds are made by bonding with electrons in the outer shell!!
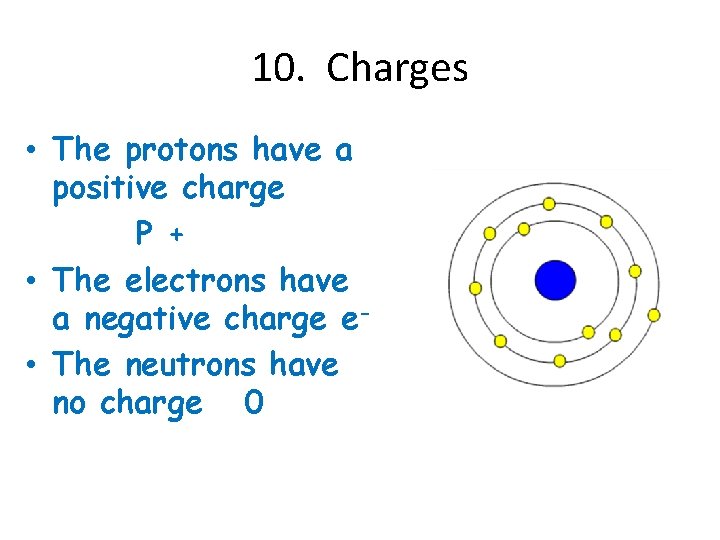
10. Charges • The protons have a positive charge P + • The electrons have a negative charge e • The neutrons have no charge 0

11. Atomic # & atomic mass • The atomic number is the # of electrons and protons • Proton # never changes • Electron # changes when bonding • Mass # is equal to atomic # + # of neutrons
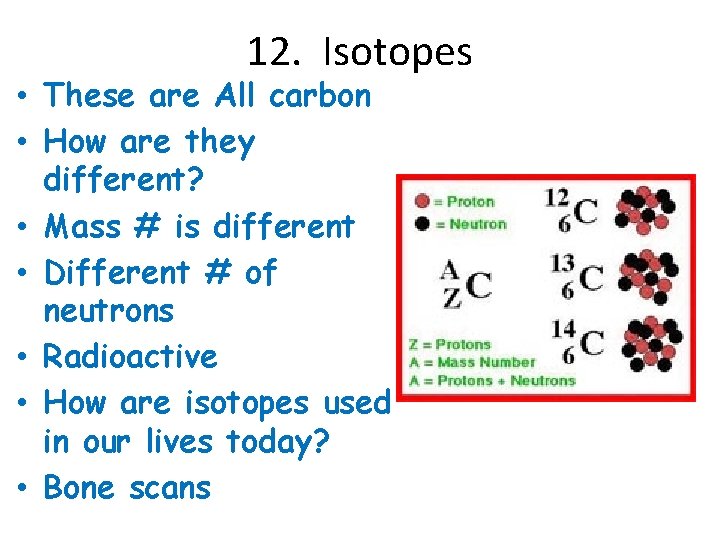
12. Isotopes • These are All carbon • How are they different? • Mass # is different • Different # of neutrons • Radioactive • How are isotopes used in our lives today? • Bone scans
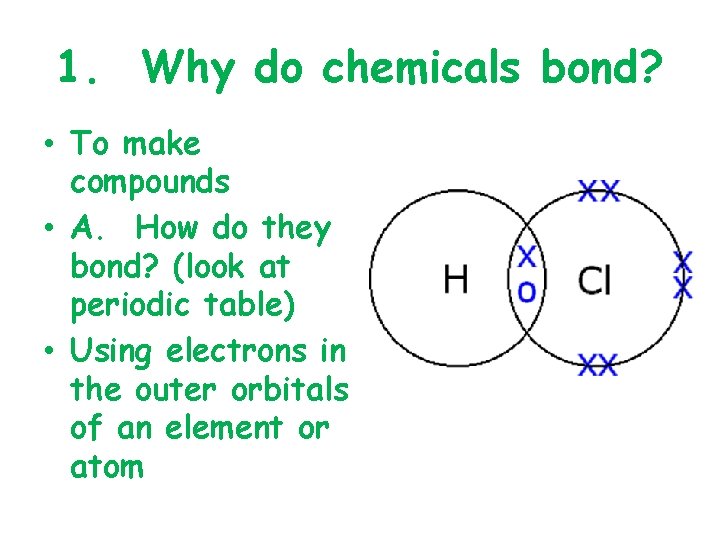
1. Why do chemicals bond? • To make compounds • A. How do they bond? (look at periodic table) • Using electrons in the outer orbitals of an element or atom

Bonds Vs TV • Bonds are the glue that hold 2 or more atoms together to make molecules which acts like a single distinct object. • A TV is made of many parts. Would the TV work if you had the screen only? ?
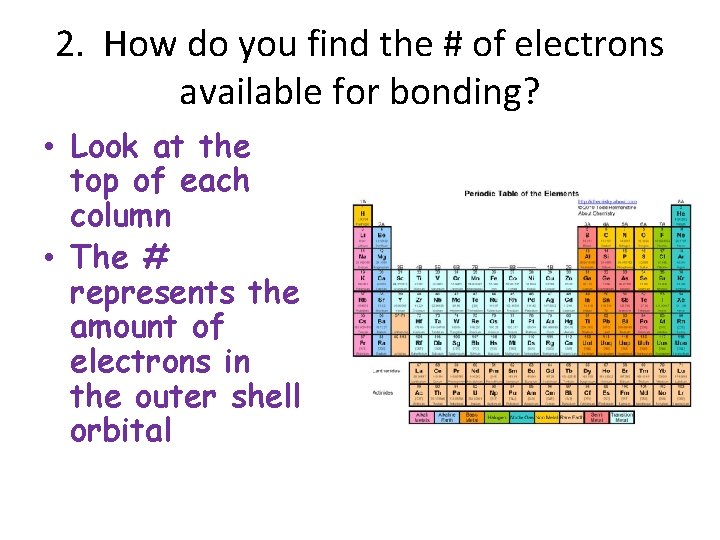
2. How do you find the # of electrons available for bonding? • Look at the top of each column • The # represents the amount of electrons in the outer shell orbital

3. Where do you find the # of electrons in an element? • On the top of the element • A. How many electrons are there in the element? • 11
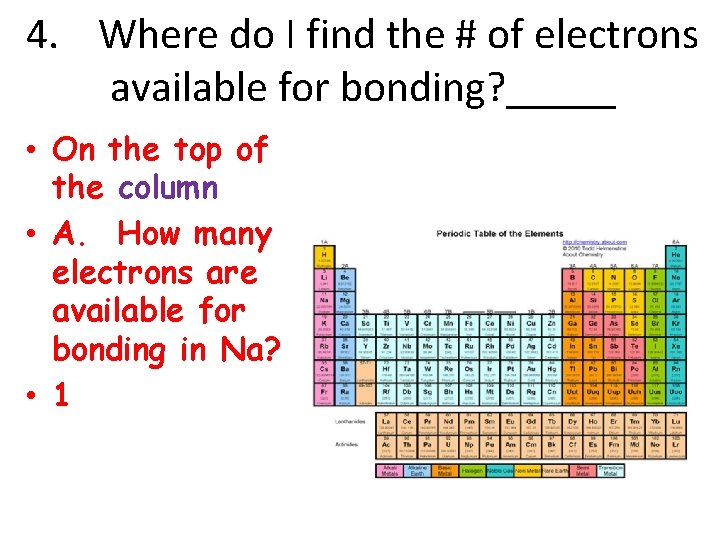
4. Where do I find the # of electrons available for bonding? _____ • On the top of the column • A. How many electrons are available for bonding in Na? • 1
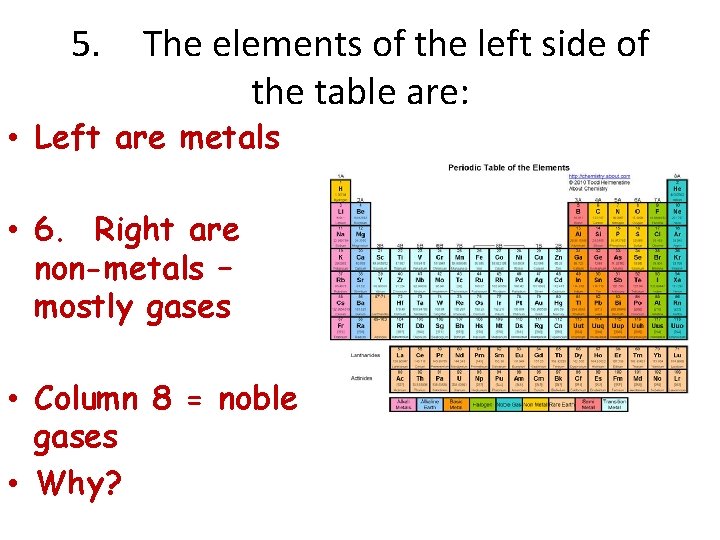
5. The elements of the left side of the table are: • Left are metals • 6. Right are non-metals – mostly gases • Column 8 = noble gases • Why?
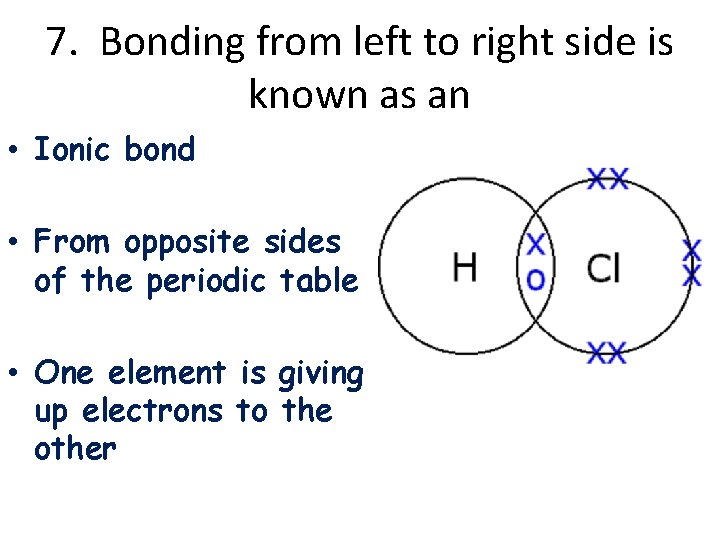
7. Bonding from left to right side is known as an • Ionic bond • From opposite sides of the periodic table • One element is giving up electrons to the other
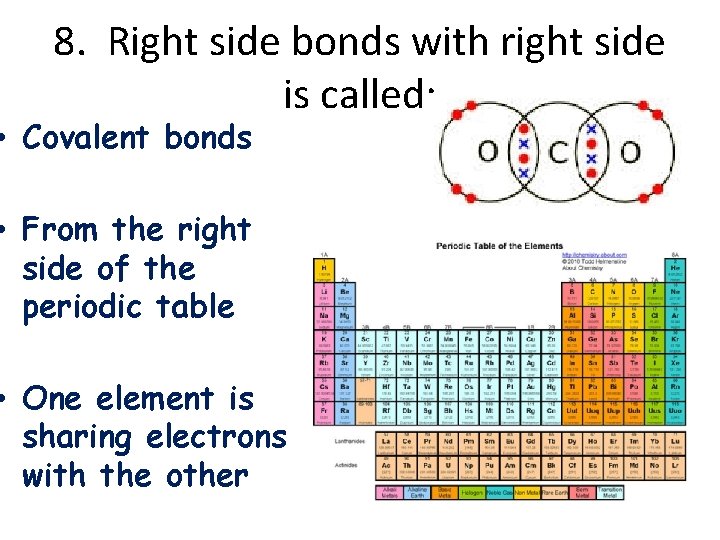
8. Right side bonds with right side is called: • Covalent bonds • From the right side of the periodic table • One element is sharing electrons with the other
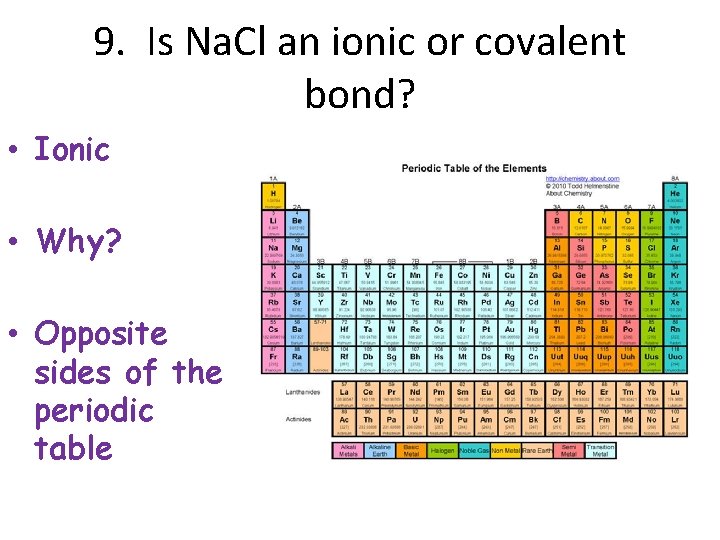
9. Is Na. Cl an ionic or covalent bond? • Ionic • Why? • Opposite sides of the periodic table
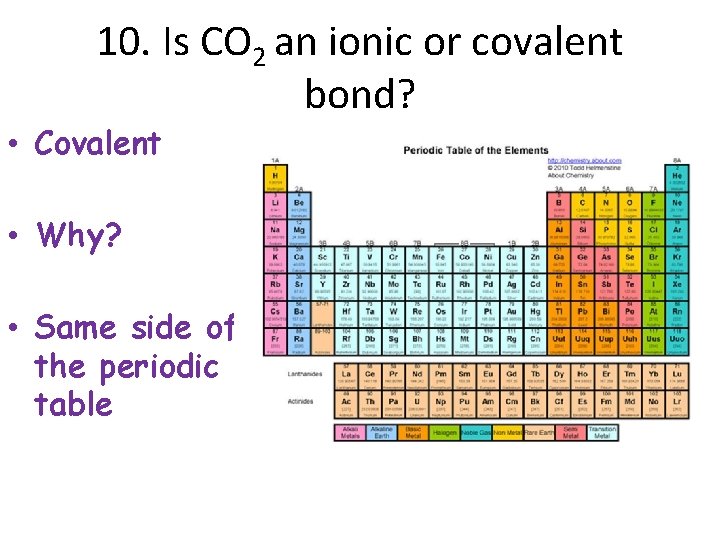
10. Is CO 2 an ionic or covalent bond? • Covalent • Why? • Same side of the periodic table
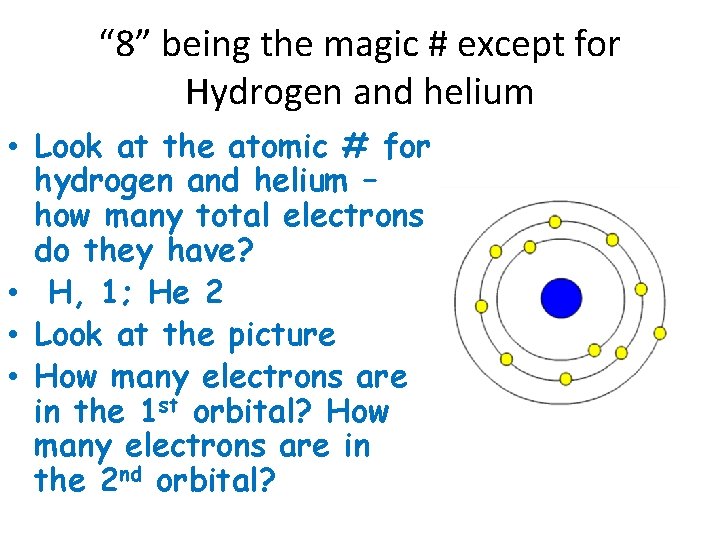
“ 8” being the magic # except for Hydrogen and helium • Look at the atomic # for hydrogen and helium – how many total electrons do they have? • H, 1; He 2 • Look at the picture • How many electrons are in the 1 st orbital? How many electrons are in the 2 nd orbital?
11. Potassium “K” is in what column (Group/Family)? • A. How many electrons are available for bonding? • 1 • B. By giving up just __ electron, it will be in its “happy” place.

12. Chlorine “Cl” is in what column? • A. How many electrons are available for bonding? • 7 • B. By taking up just __ electron, it will be in its “happy” place
13. Carbon “C” is in what column? • A. How many electrons are available for bonding? • 4 • B. By giving up or taking just sharing electrons, it will be in its “happy” place
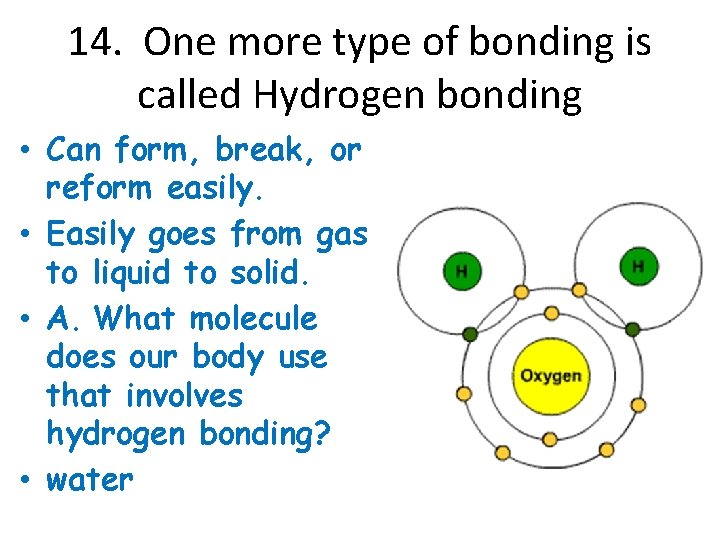
14. One more type of bonding is called Hydrogen bonding • Can form, break, or reform easily. • Easily goes from gas to liquid to solid. • A. What molecule does our body use that involves hydrogen bonding? • water
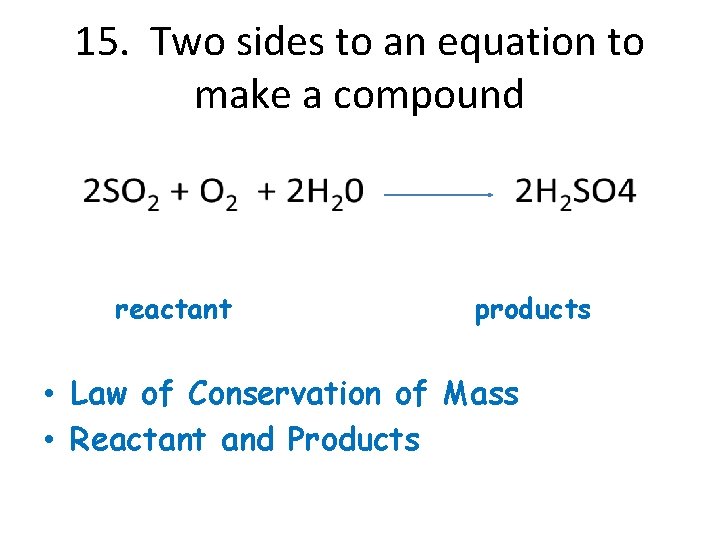
15. Two sides to an equation to make a compound reactant products • Law of Conservation of Mass • Reactant and Products
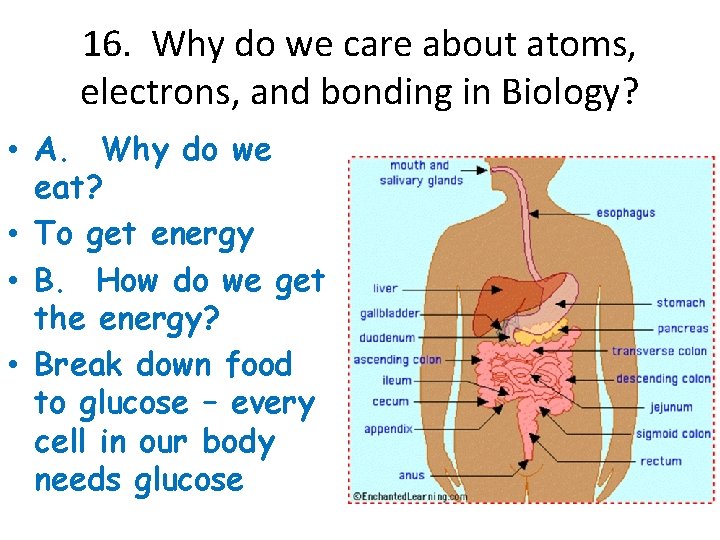
16. Why do we care about atoms, electrons, and bonding in Biology? • A. Why do we eat? • To get energy • B. How do we get the energy? • Break down food to glucose – every cell in our body needs glucose
17. What are the most important elements to Biology? • CHNOPS • Carbon is in all living things (carbon backbone) • H 2 O is in all living things • Proteins have Nitrogen and sulfur • Phosphorus is in phospholipids
- Slides: 30