Matter Study Guide 5 th Grade Science Matter






- Slides: 15

Matter Study Guide 5 th Grade Science

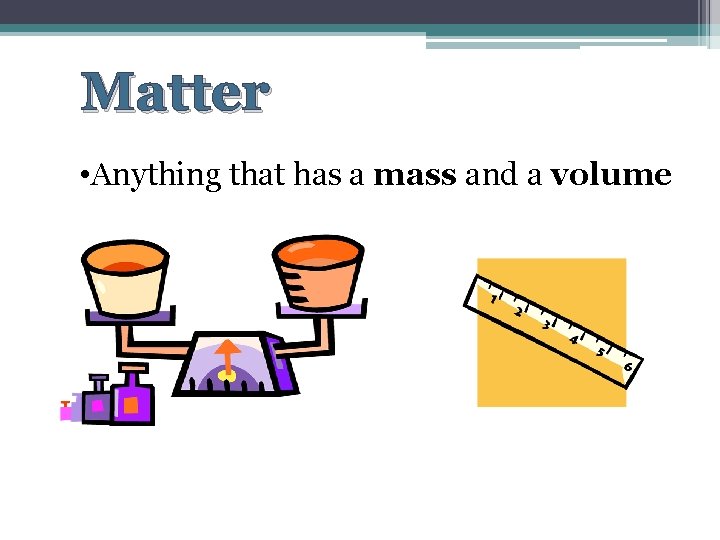
Matter • Anything that has a mass and a volume

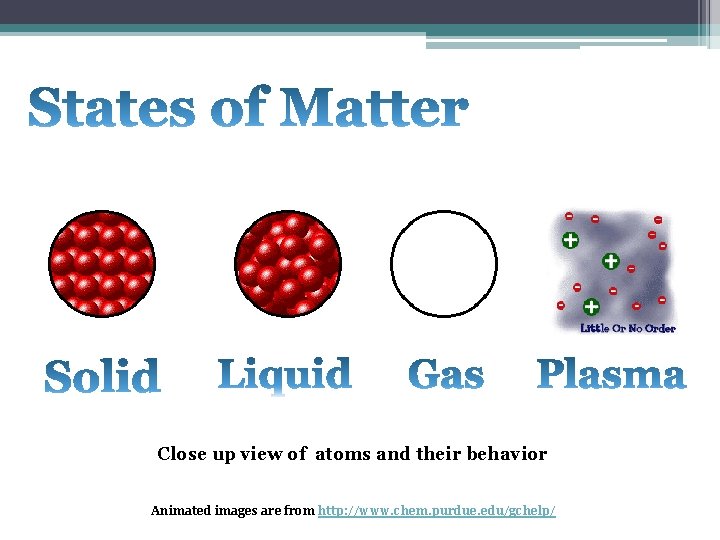
Close up view of atoms and their behavior Animated images are from http: //www. chem. purdue. edu/gchelp/

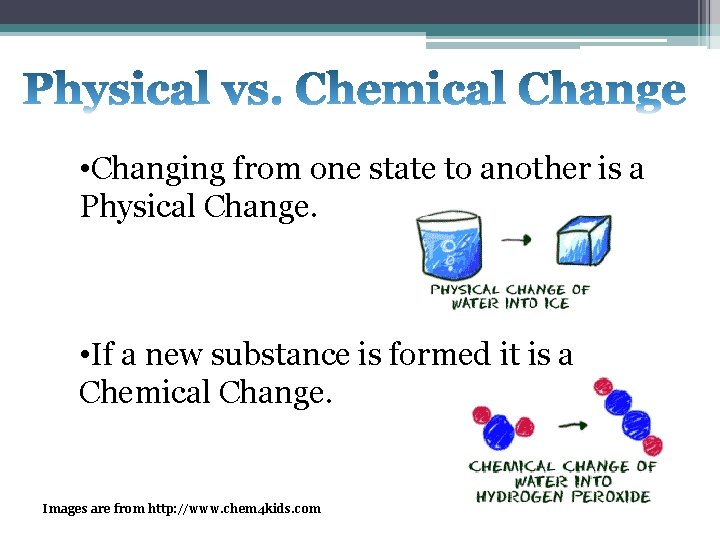
• Changing from one state to another is a Physical Change. • If a new substance is formed it is a Chemical Change. Images are from http: //www. chem 4 kids. com

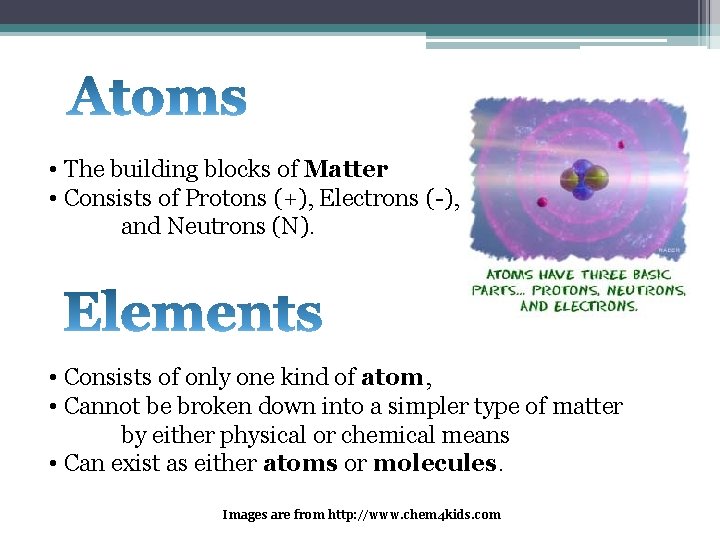
• The building blocks of Matter • Consists of Protons (+), Electrons (-), and Neutrons (N). • Consists of only one kind of atom, • Cannot be broken down into a simpler type of matter by either physical or chemical means • Can exist as either atoms or molecules. Images are from http: //www. chem 4 kids. com

• A molecule consists of two or more atoms of the same element, or different elements, that are chemically bound together. • In the animation above, two nitrogen atoms (N + N = N 2) make one Nitrogen molecule.

• Atoms of two or more different elements bound together. • Can be separated into elements chemically, but not physically. In the animation above, water (H 20) is a compound made of Hydrogen and Oxygen. Animated images and notes from http: //www. chem. purdue. edu/gchelp/atoms/elements. html

• Solutions are groups of molecules that are mixed up in a completely even distribution. • Uniform Distribution. • Example: Sugar and Water Images are from http: //www. chem 4 kids. com

• The substance to be dissolved. • The one doing the dissolving. Images are from http: //www. chem 4 kids. com


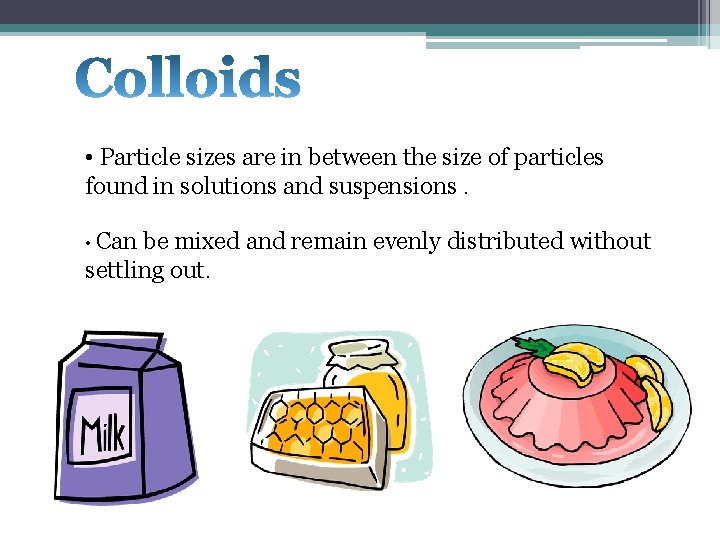
• Particle sizes are in between the size of particles found in solutions and suspensions. • Can be mixed and remain evenly distributed without settling out.

• They are substances held together by physical forces, not chemical. • Can be separated physically. • Solutions are also mixtures. • The substances are not uniformly mixed. • Example: Sand in a glass of water. Images are from http: //www. chem 4 kids. com

• Are heterogeneous mixtures consisting of parts that are visible to the naked eye. • Substances will settle over time. Example: the ingredients in salad dressing

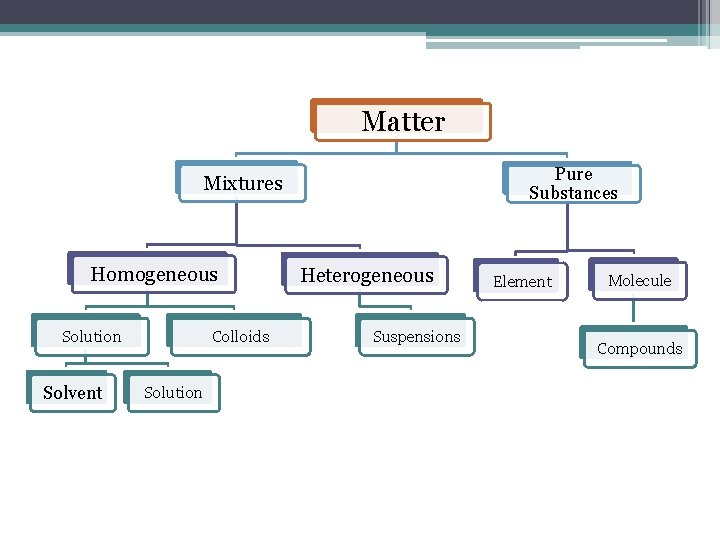
Matter Pure Substances Mixtures Homogeneous Colloids Solution Solvent Solution Heterogeneous Suspensions Element Molecule Compounds
