Matter Section 3 Changes of Matter Preview Key








- Slides: 13

Matter Section 3: Changes of Matter Preview • Key Ideas • Bellringer • Physical Changes • Chemical Changes • Breaking Down Mixtures and Compounds

Matter Section 3 Key Ideas 〉 Why is getting a haircut an example of a physical change? 〉 Why is baking bread an example of a chemical change? 〉 How can mixtures and compounds be broken down?

Matter Section 3 Bellringer Matter can go through both physical and chemical changes and can exist as mixtures and compounds. See how matter interacts when Jordan makes oatmeal bread. 1. To make bread, Jordan must do several things before it is ready to be baked. For each step, decide whether a physical or a chemical change occurs, or whether a mixture or compound is formed. Circle the correct answer. a. stir flour and dry oatmeal mixture formed compound formed b. heat the water physical change chemical change c. melt the shortening physical change chemical change d. beat the eggs physical change chemical change e. blend molasses with water mixture formed

Matter Section 3 Bellringer, continued 2. Jordan accidentally measures sugar instead of flour and stirs the dry oatmeal with the sugar. Is a mixture or a compound created? How can the two ingredients be separated so that the bread is not ruined? 3. The recipe calls for baking powder and water. When these two ingredients come into contact with each other, gas is given off. This helps the bread rise. Is this an example of a physical change or a chemical change? Explain your answer.

Matter Section 3 Physical Changes 〉 Why is getting a haircut an example of a physical change? 〉 A physical change affects one or more properties of a substance without changing the identity of the substance. • physical change: a change of matter from one form to another without a change in chemical properties

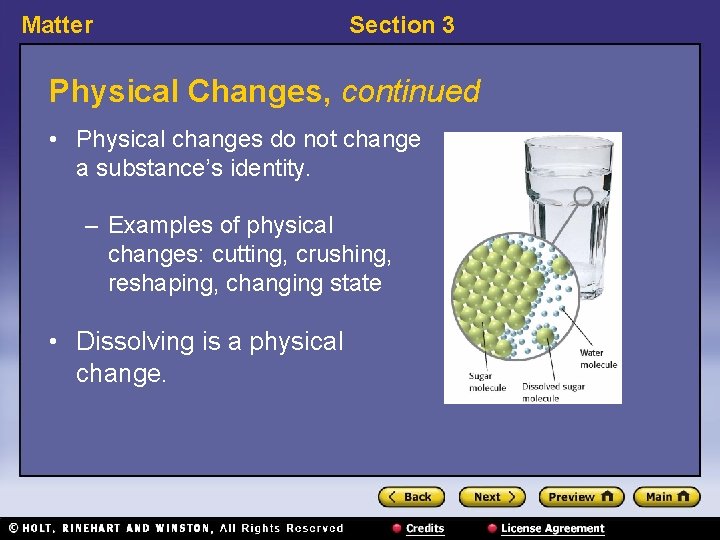
Matter Section 3 Physical Changes, continued • Physical changes do not change a substance’s identity. – Examples of physical changes: cutting, crushing, reshaping, changing state • Dissolving is a physical change.

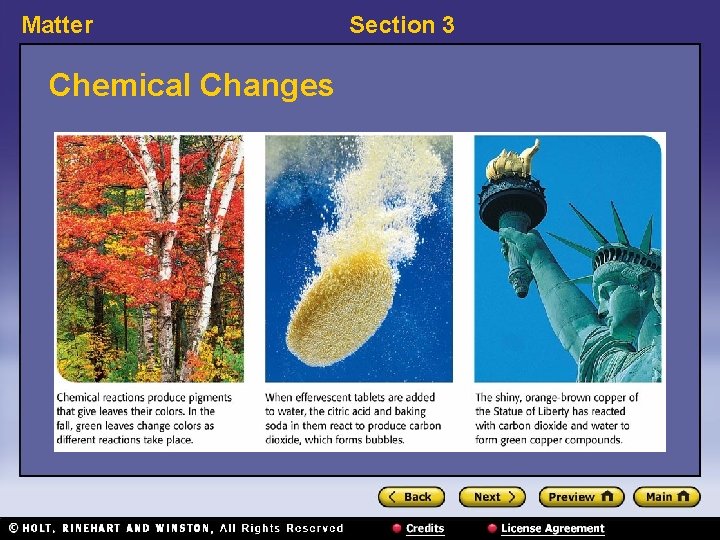
Matter Section 3 Chemical Changes 〉 Why is baking bread an example of a chemical change? 〉 A chemical change happens when one or more substances are changed into entirely new substances that have different properties. • chemical change: a change that occurs when one or more substances change into entirely new substances with different properties

Matter Chemical Changes Section 3

Matter Section 3 Chemical Changes, continued • Chemical changes happen everywhere. – Examples of chemical changes: burning, rusting, digesting, decomposing • Chemical changes form new substances. • Chemical changes can be detected. – Signs include: change of color, change of smell, fizzing, production of heat, production of sound, production of light • Chemical changes cannot be reversed by physical changes.

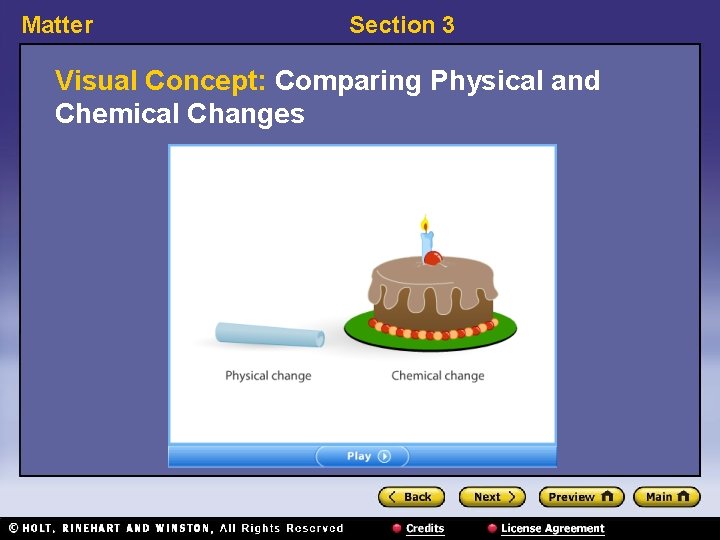
Matter Section 3 Visual Concept: Comparing Physical and Chemical Changes

Matter Section 3 Breaking Down Mixtures and Compounds 〉 How can mixtures and compounds be broken down? 〉 Mixtures can be separated by physical changes, but compounds must be broken down by chemical changes.

Matter Section 3 Breaking Down Mixtures and Compounds, continued • Mixtures can be physically separated. • Examples of separating a mixture: – Separating saltwater into its parts by heating it: When the water evaporates, the salt remains. – Using a distillation device to heat a mixture whose components have different boiling points: The component that boils and evaporates first separates from the mixture. – Using a centrifuge: The mixture spins rapidly until the components separate.

Matter Section 3 Breaking Down Mixtures and Compounds, continued • Some compounds can be broken down through chemical changes. • Examples of separating a compound: – When mercury(II) oxide is heated, it breaks down into the elements mercury and oxygen. – When a current is passed through melted table salt, the elements sodium and chlorine are produced. – When you open a bottle of soda, carbonic acid in the soda breaks down into carbon dioxide and water.