MATTER PURE SUBSTANCE ELEMENT COMPOUND MIXTURE HOMOGENEOUS SOLUTION
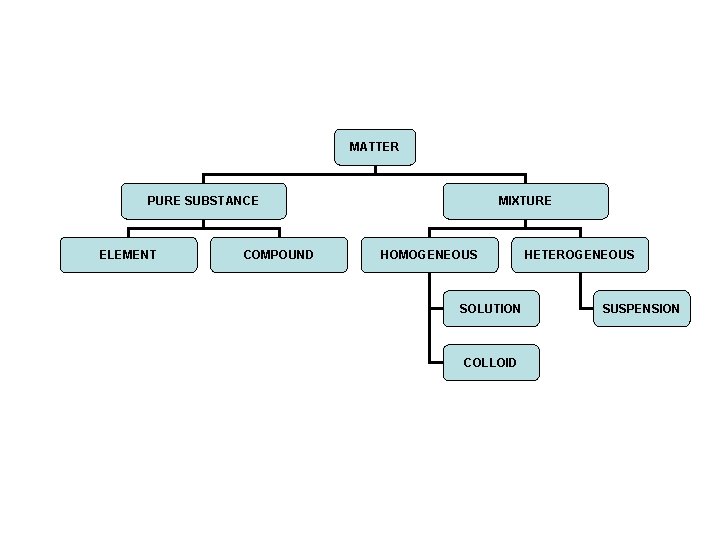
MATTER PURE SUBSTANCE ELEMENT COMPOUND MIXTURE HOMOGENEOUS SOLUTION COLLOID HETEROGENEOUS SUSPENSION

Pure substance 1. Only one kind of material 2. Definite properties that are the same throughout 3. Types a) elements b) compounds
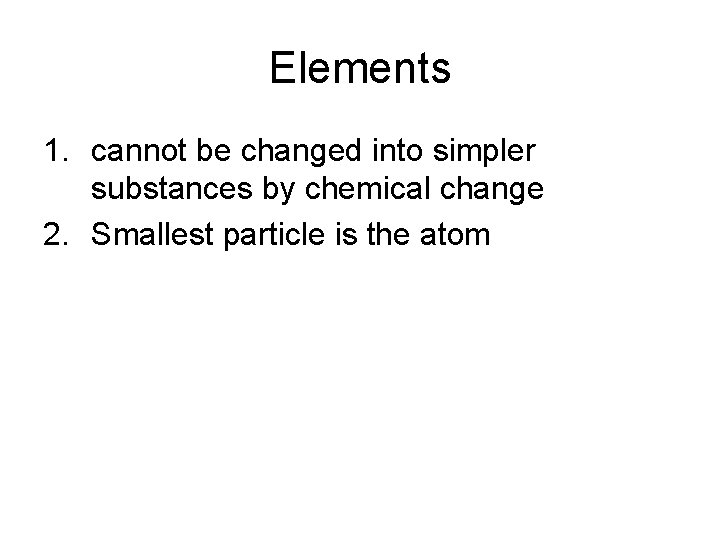
Elements 1. cannot be changed into simpler substances by chemical change 2. Smallest particle is the atom

Compounds 1. pure substances made up of one or more elements chemically combined 2. Can be broken down into simpler substances by heat, electricity or other means 3. Properties may be different than the elements which make up the compound

Mixture • • Each substance keeps its own specific properties Not the same throughout Not chemically combined Can be separated by physical means (filtration or distillation)

Heterogeneous Mixture • • Least mixed Large particles Suspension Examples: concrete, oil & vinegar

Homogeneous Mixtures 1. Appears the same throughout 2. Particles do not settle when mixture allowed to stand 3. Types a) solution b) colloid

Solution • • One substance dissolves in another Solute: substance that dissolves Solvent: substance that does the dissolving Not easily physically separated by filtering Particles too small to scatter light Looks clear and transparent Every part tastes the same

Colloid • Particles are mixed together but not dissolved – e. g. milk • Particles are permanently suspended • Will NOT separate on standing • Usually looks cloudy – scatters light • Medium sized particles

Types of mixtures
- Slides: 10