Matter Properties Chapter 3 Matter Anything that has













- Slides: 16

Matter – Properties Chapter 3

Matter – Anything that has mass and occupies space – Mass is measured in grams or kilograms – Space or capacity or volume is measured in liters or cm 3.

Substances • Matter that has the same composition all the way through. • Pure • Elements or Compounds • H 2 O, Na. Cl, O 2, He

Formulas = Identity • Element = 1 upper case letter • Compound = 2 or more upper case letters

Properties of Matter Section 3. 1

Physical Properties • Describe the appearance and behavior of matter -Words: color, texture, luster, odor, solid, liquid, gas. Qualitative -Measurements: a number and a unit. Quantitative

Physical Properties • Use them to identify matter!

Intensive properties – are independent of the sample size. – Physical Constants are quantitative intensive properties. They have a number and a unit. • Density, freezing point, and melting point • Solubility in water (g/ml) • Heat of fusion, Heat of vaporization, Specific heat capacity

Extensive Properties • Extensive properties depend on the amount of matter in the sample. • Mass & Volume

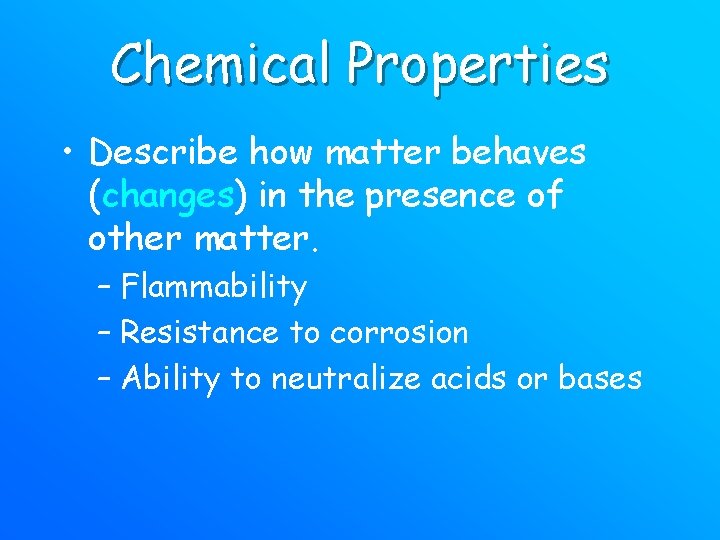
Chemical Properties • Describe how matter behaves (changes) in the presence of other matter. – Flammability – Resistance to corrosion – Ability to neutralize acids or bases

Properties of Copper • • Physical Prop. Reddish brown Shiny Malleable Ductile Good Conductor Density = 8. 92 g/cm 3 MP = 1085 C BP = 2570 C Chemical Prop. • Reacts to form green copper carbonate • Forms a deep blue solution when in contact with NH 3 • Forms new substances with HNO 3.

Phases • Solid – Definite volume and definite shape. • Liquid – Definite volume and indefinite shape. (Takes the shape of its container. ) • Gas – Indefinite volume and indefinite shape. (Takes the shape and volume of its container. )

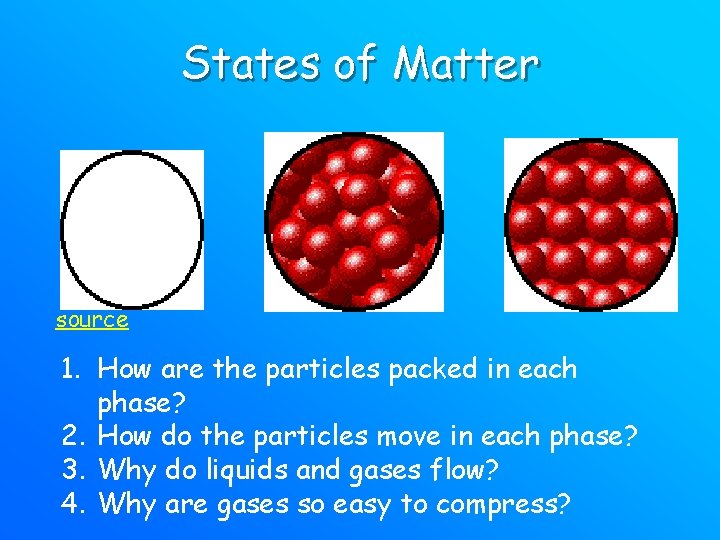
States of Matter source 1. How are the particles packed in each phase? 2. How do the particles move in each phase? 3. Why do liquids and gases flow? 4. Why are gases so easy to compress?

th 4 Phase of matter • Plasma – exists in stars, lightning • Electrons are stripped from atoms.

Phases of Matter • Solid • Liquid • Gas • TIGER

Notation of Phase • After the formula, use the letters: – s for solid Al(s) – g for gas H 2 O(g) – l for liquid CH 3 OH(l) – aq means dissolved in water The state or phase of matter is a physical property! Na. Cl(aq)