Matter Properties Change Unit One A Matter l


























- Slides: 34

Matter: Properties & Change Unit One

A. Matter l Matter – anything that has mass and takes up space l l Everything around us Chemistry – the study of matter and the changes it undergoes

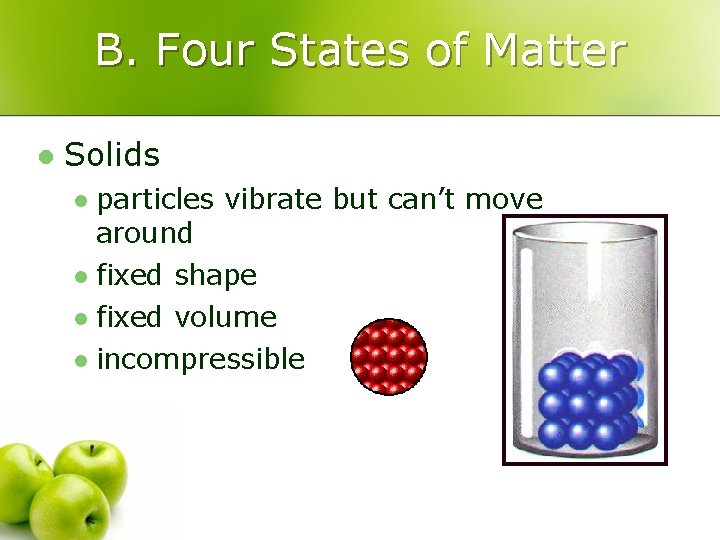
B. Four States of Matter l Solids particles vibrate but can’t move around l fixed shape l fixed volume l incompressible l

B. Four States of Matter l Liquids particles can move around but are still close together l variable shape l fixed volume l l Virtually incompressible

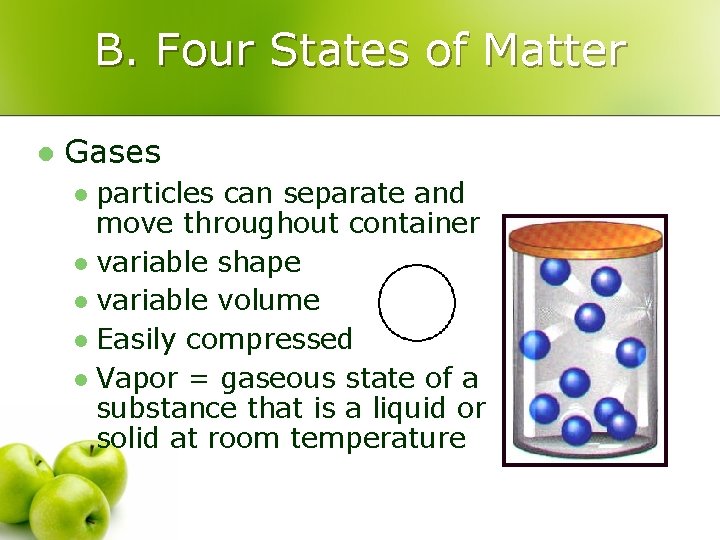
B. Four States of Matter l Gases particles can separate and move throughout container l variable shape l variable volume l Easily compressed l Vapor = gaseous state of a substance that is a liquid or solid at room temperature l

B. Four States of Matter l Plasma particles collide with enough energy to break into charged particles (+/-) l gas-like, variable shape & volume l stars, fluorescent light bulbs, TV tubes l

II. Properties & Changes in Matter l Extensive vs. Intensive l Physical vs. Chemical

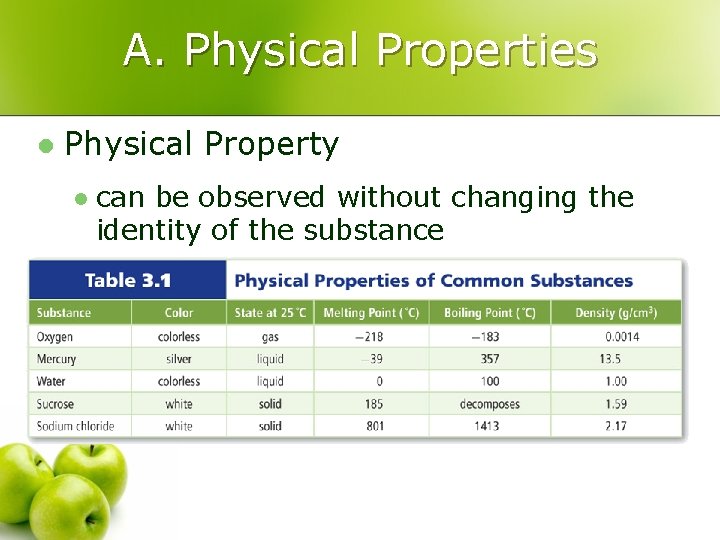
A. Physical Properties l Physical Property l can be observed without changing the identity of the substance

A. Physical Properties l Physical properties can be described as one of 2 types: l Extensive Property l l depends on the amount of matter present (example: length) Intensive Property l depends on the identity of substance, not the amount (example: scent)

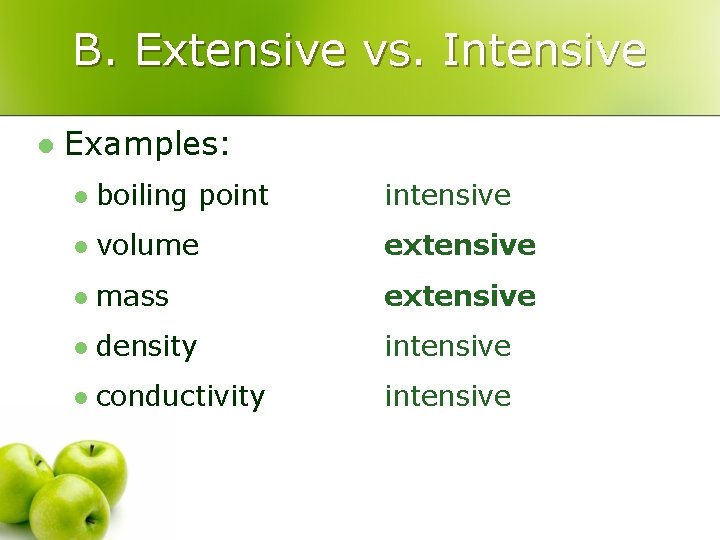
B. Extensive vs. Intensive l Examples: l boiling point intensive l volume extensive l mass extensive l density intensive l conductivity intensive

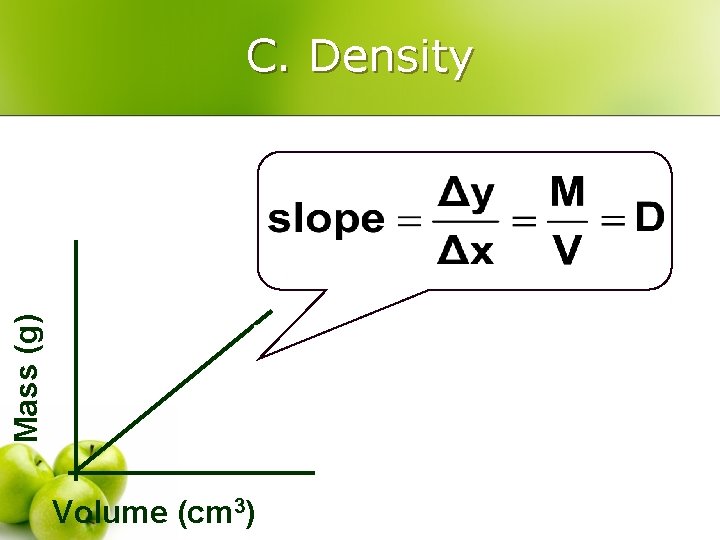
C. Density – a physical property l l Derived units = Combination of base units Volume (m 3 or cm 3 or m. L) l l length Or measured using a graduated cylinder 1 cm 3 = 1 m. L 1 dm 3 = 1 L Ø Density (kg/m 3 or g/cm 3 or g/m. L) w mass per volume M D= V

Mass (g) C. Density Volume (cm 3)

C. Density l An object has a volume of 825 cm 3 and a density of 13. 6 g/cm 3. Find its mass. GIVEN: WORK: V = 825 cm 3 D = 13. 6 g/cm 3 M=? M = DV M = (13. 6 g/cm 3)(825 cm 3) M = 11, 220 g M = 11, 200 g

C. Density l A liquid has a density of 0. 87 g/m. L. What volume is occupied by 25 g of the liquid? GIVEN: WORK: D = 0. 87 g/m. L V=? M = 25 g V=M D V = 25 g 0. 87 g/m. L V = 29 m. L = 28. 736 m. L

D. Chemical Properties l Chemical Property l describes the ability of a substance to undergo changes in identity

E. Physical vs. Chemical Properties l Examples: l melting point physical l flammable chemical l density physical l magnetic physical l tarnishes in air chemical

F. Physical Changes l l Physical Change l changes the form of a substance without changing its identity l properties remain the same Examples: cutting a sheet of paper, breaking a crystal, all phase changes

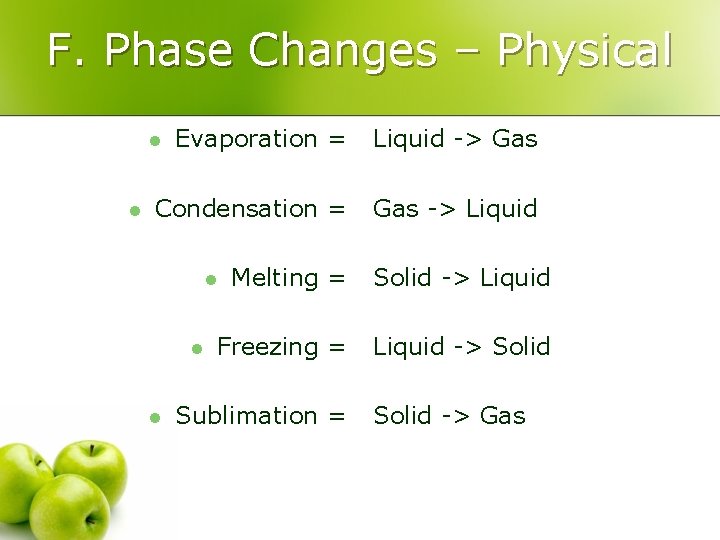
F. Phase Changes – Physical Evaporation = Liquid -> Gas Condensation = Gas -> Liquid l l Melting = Solid -> Liquid Freezing = Liquid -> Solid l l l Sublimation = Solid -> Gas

G. Chemical Changes l Process that involves one or more substances changing into a new substance Commonly referred to as a chemical reaction l New substances have different compositions and properties from original substances l

G. Chemical Changes l Signs of a Chemical Change l change in color or odor l formation of a gas l formation of a precipitate (solid) l change in light or heat

H. Physical vs. Chemical Changes l Examples: l rusting iron chemical l dissolving in water physical l burning a log chemical l melting ice physical l grinding spices physical

What Type of Change?

What Type of Change?

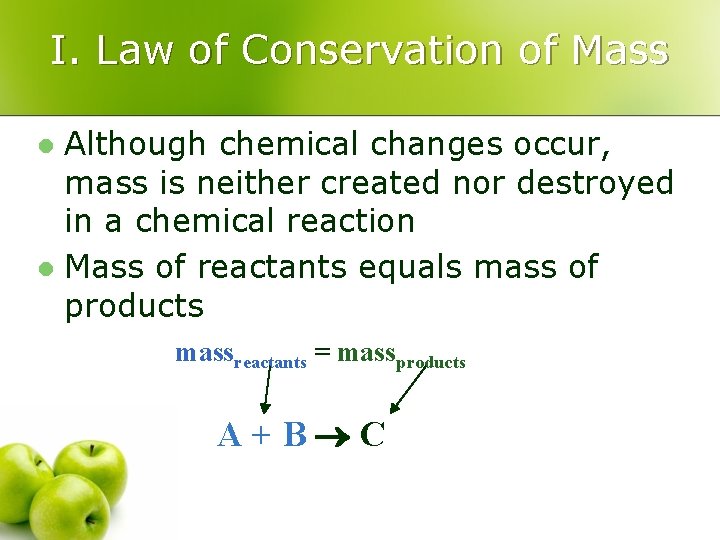
I. Law of Conservation of Mass Although chemical changes occur, mass is neither created nor destroyed in a chemical reaction l Mass of reactants equals mass of products l massreactants = massproducts A+B C

I. Conservation of Mass l In an experiment, 10. 00 g of red mercury (II) oxide powder is placed in an open flask and heated until it is converted to liquid mercury and oxygen gas. The liquid mercury has a mass of 9. 26 g. What is the mass of the oxygen formed in the reaction? GIVEN: WORK: 10. 00 g = 9. 86 g + moxygen Mercury (II) oxide mercury + oxygen Mmercury(II) oxide = 10. 00 g Moxygen = (10. 00 g – 9. 86 Mmercury = 9. 86 g Mmercury(II) oxide = 10. 00 g Moxygen =? Mmercury = 9. 26 Moxygen = 0. 74 g Moxygen = ? massreactants = massproducts g)

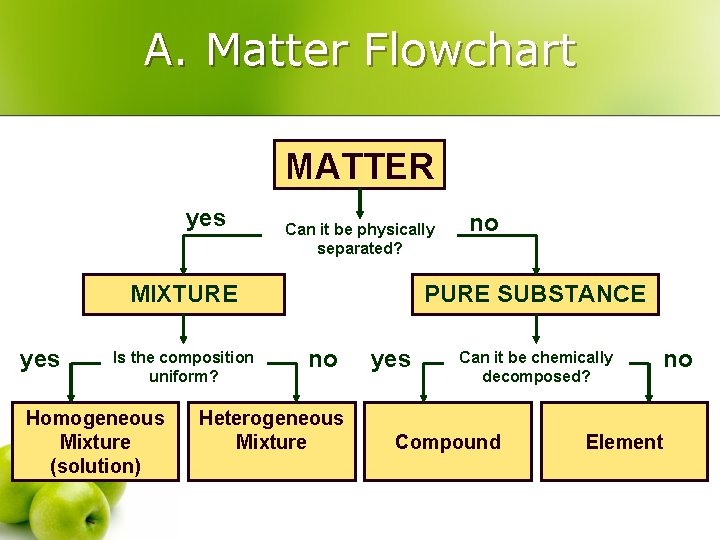
III. Classification of Matter l Matter Flowchart l Pure Substances l Mixtures

A. Matter Flowchart MATTER yes Can it be physically separated? MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no PURE SUBSTANCE no Heterogeneous Mixture yes Can it be chemically decomposed? Compound no Element

A. Matter Flowchart l Examples: l graphite element l pepper hetero. mixture l sugar (sucrose) compound l paint hetero. mixture l soda solution

B. Pure Substances l Element composed of identical atoms l EX: copper wire, aluminum foil l

B. Pure Substances l Compound l composed of 2 or more elements in a fixed ratio l properties differ from those of individual elements l EX: table salt (Na. Cl)

C. Mixtures l Variable combination of 2 or more pure substances. Heterogeneous Homogeneous

C. Mixtures l Solution homogeneous l very small particles don’t settle l EX: rubbing alcohol l

C. Mixtures l Heterogeneous medium-sized to large-sized particles l particles may or may not settle l EX: milk, freshsqueezed lemonade l

C. Mixtures l Examples: l Answers: l tea l Solution l muddy water l Heterogeneous l fog l Heterogeneous l saltwater l Solution l Italian salad dressing l Heterogeneous