Matter Properties and Changes Physical Properties Can be

Matter Properties and Changes
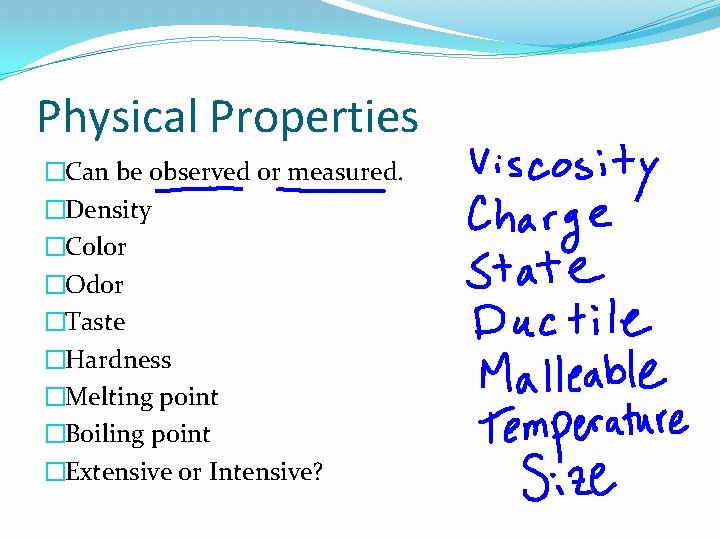
Physical Properties �Can be observed or measured. �Density �Color �Odor �Taste �Hardness �Melting point �Boiling point �Extensive or Intensive?
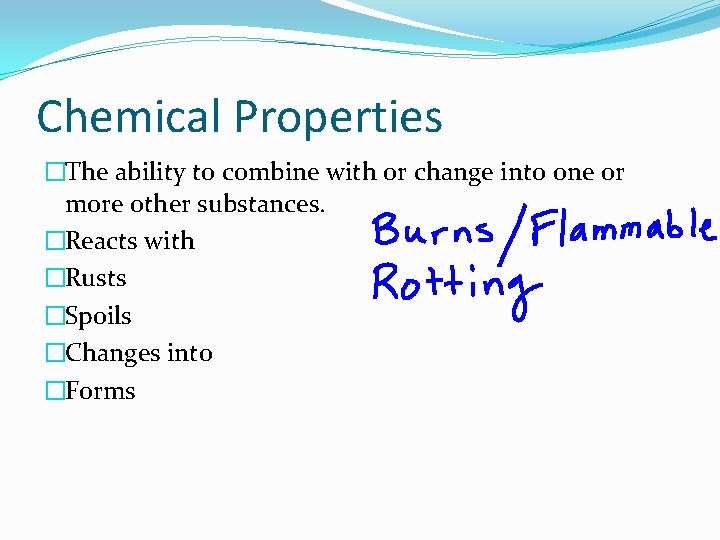
Chemical Properties �The ability to combine with or change into one or more other substances. �Reacts with �Rusts �Spoils �Changes into �Forms

States of Matter �Solid – definite shape and volume �Liquid – definite volume, flows, takes shape of container �Gas (Vapor) – flows, fills volume of container �Plasma – in stars, the Sun, lightning
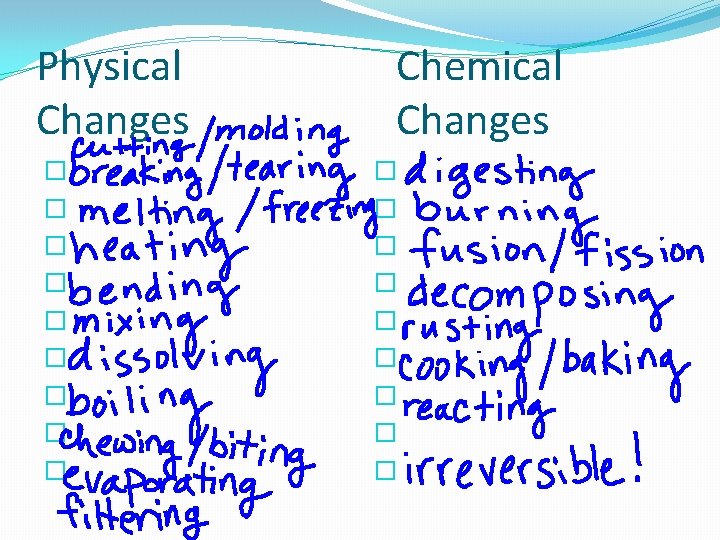
Physical Changes � � � � � Chemical Changes � � � � �
Signs of a Chemical Change/Reaction �Color change �Odor �Bubbles / production of a new gas �Precipitate / formation of a new solid from combining two solutions �Heat (energy) being absorbed or given off / becoming cold or hot �Irreversible / reactants -> products

Matter: Mixture or Substance? �Mixture: Homogeneous or Heterogeneous? �Homogeneous = uniform composition (solutions, alloys) �Can be physically separated into multiple substances �Substance: Element or Compound? �Substance = definite composition �Element = found on the Periodic Table �Compound = can be chemically separated into Elements
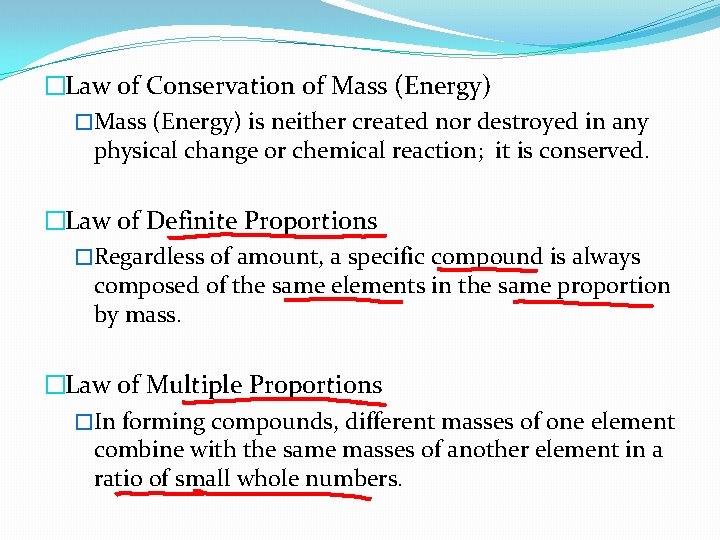
�Law of Conservation of Mass (Energy) �Mass (Energy) is neither created nor destroyed in any physical change or chemical reaction; it is conserved. �Law of Definite Proportions �Regardless of amount, a specific compound is always composed of the same elements in the same proportion by mass. �Law of Multiple Proportions �In forming compounds, different masses of one element combine with the same masses of another element in a ratio of small whole numbers.
- Slides: 8