MATTER Properties and Changes Matter is anything that








- Slides: 14

MATTER: Properties and Changes

Matter is anything that has mass and takes up space.

Physical Properties • Can be observed or measured without changing the identity of the material A. COLOR B. SHAPE C. LENGTH D. MASS

Physical Properties (cont’d) E. VOLUME – mass/ quantity F. DENSITY – the amount of mass for a given volume G. MELTING/ BOILING POINTS - temperature at which material melts/ H. a LUSTER - shinyboils

Physical Properties • Can be observed or measured without changing the identity of the material A. COLOR B. SHAPE C. LENGTH D. MASS

Physical Properties (cont’d) E. VOLUME – mass/ quantity F. DENSITY – the amount of mass for a given volume G. MELTING/ BOILING POINTS - temperature at which material melts/ H. a LUSTER - shinyboils

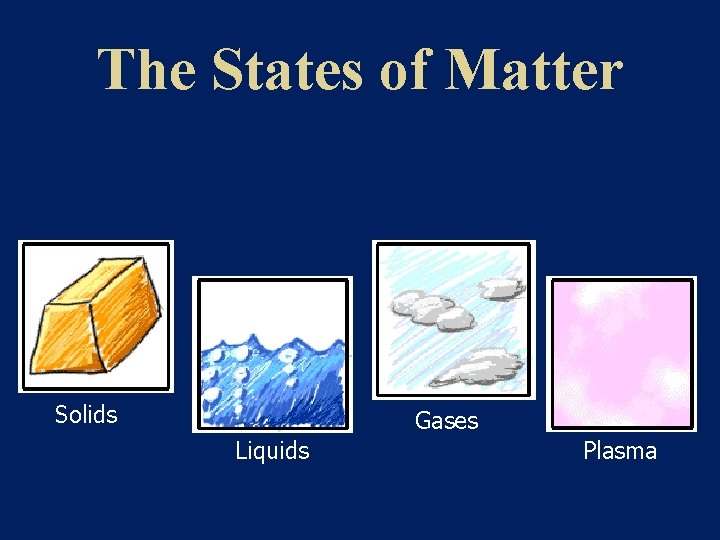
The States of Matter Solids Gases Liquids Plasma

Solids • Solids hold their own shape. • Solids have weight. • Solids take up space. • Are packed tightly together • Have very little energy

Liquids • Liquids take the shape of their container. • Liquids have weight. • Liquids take up space. • Are loosely packed • Have medium energy levels

Gases • Gases spread out to fill the entire space given. • Gases have weight. • Gases take up space. • Move freely • Have LOTS of energy

Changes in the States of Matter • Changes in the states of matter are physical changes. • A. MELTING – a solid to a liquid • B. CONDENSATION – gas/vapor into a liquid • C. FREEZING – use cold for liquid to form a solid • D. EVAPORATION – liquid into

Changes in the States of Matter (cont’d) • F. VAPORIZATION – a solid/liquid turns into gas/ vapor • G. BOILING – a liquid boils/ begins to vaporize • H. LIQUIFICATION – cooling air until it forms a liquid

Chemical Properties • Any characteristic that gives a substance the ability to undergo a change that results in a new substance REACTS WITH OXYGEN FLAMMA BILITY REACTS WITH WATER REACTS WITH

Clues for Identifying Chemical Changes • • • A new color appears Heat or light is given off Bubbles form The change is not easily reversible The smell of a new gas might be detected New and different properties are observed