Matter Properties and Changes Chapter 3 Pages 70








































- Slides: 55

Matter Properties and Changes Chapter 3 Pages 70 -90

Matter Properties and Changes Learning Goals I know the 3 states of matter and their characteristics. I can distinguish between physical and chemical properties. I understand what the law of conservation of mass means. I can identify a mixture as homogeneous or heterogeneous. I can list and describe several techniques used to separate mixtures.

What is Matter? Matter- anything that has mass and takes up space! Pure substance- any matter with a uniform and unchanging composition. Examples of Pure Substances Water – H 2 O Table salt – sodium chloride

Pure Substances Mass A measure of the amount of matter. Atom The smallest unit of an element that maintains the properties of that element.

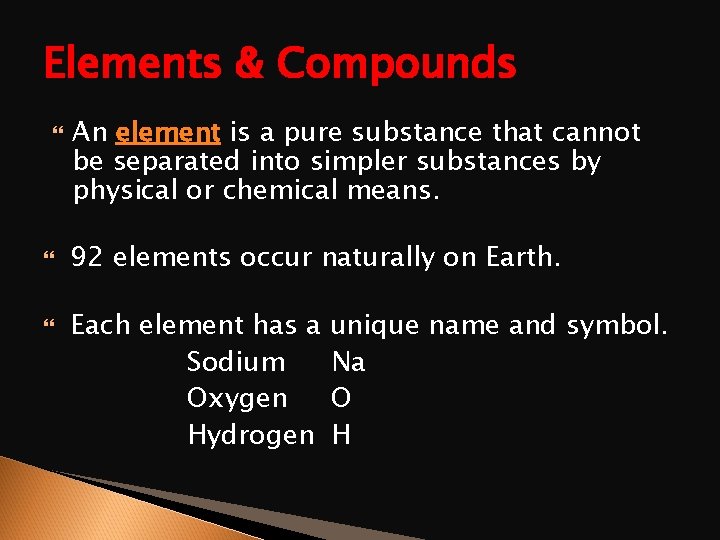
Elements & Compounds An element is a pure substance that cannot be separated into simpler substances by physical or chemical means. 92 elements occur naturally on Earth. Each element has a Sodium Oxygen Hydrogen unique name and symbol. Na O H

Elements and Compounds The periodic table organizes the elements into a grid of horizontal rows called periods and vertical columns called groups. A compound is a made up of two or more elements combined chemically. Table salt (Na. Cl) is a compound made up of the elements Na and Cl. 2 Na + Cl 2 2 Na. Cl Reactants Products

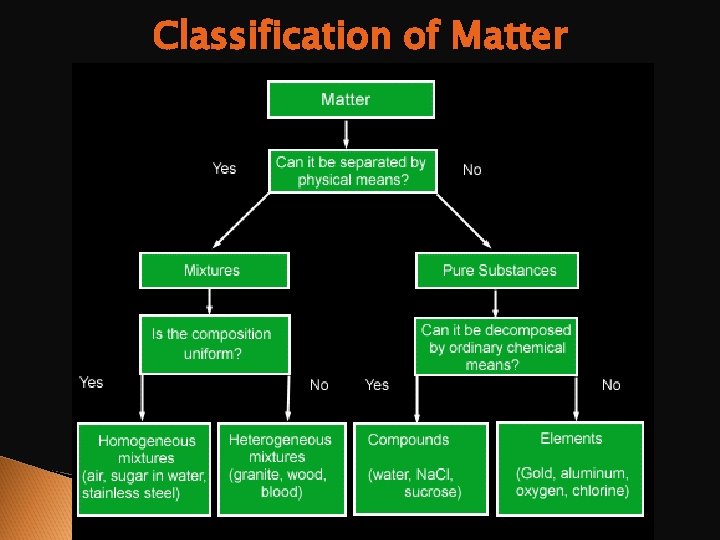
Mixtures Homogeneous mixture: uniform throughout, cannot distinguish the components in the mixture. Heterogeneous mixture: not uniform, each component in mixture remains distinct.

Mixtures Heterogeneous Mixture → Homogeneous Mixture

Solutions Homogeneous mixtures also called solutions. Examples of solutions (homogeneous mixtures): 1. Liquid-liquid solution: sodium chloride, Na. Cl added to water to form a salt solution. 2. Gas-gas solution: the air we breathe is a solution of oxygen, nitrogen and argon.

Solutions 3. Solid-solid solutions: Example: metal alloys of bronze (copper and tin solution) and steel (iron and carbon solution).

Confusing? ? Get it straight now Elements – contain only one type of atom Atoms – smallest particle that retains the identity of an element Compounds & molecules – Two or more atoms combined chemically in definite proportions. Mixture – Two or more compounds that combine but NOT CHEMICALLY. Solution – A homogeneous mixture.

Classification of Matter

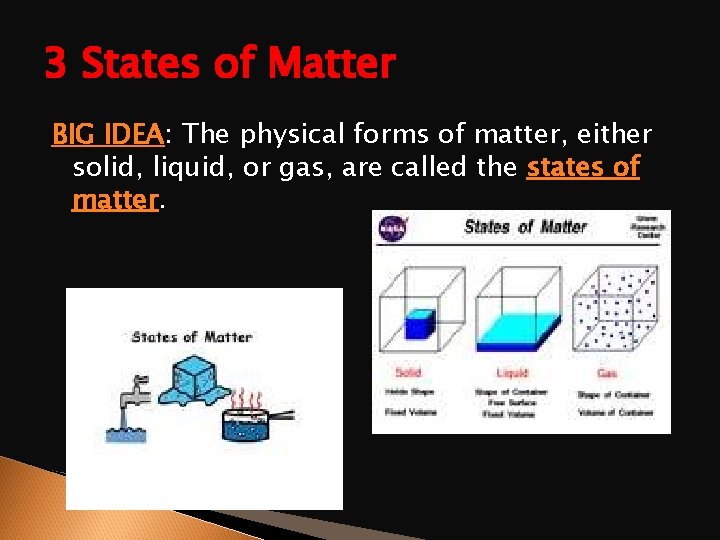
3 States of Matter BIG IDEA: The physical forms of matter, either solid, liquid, or gas, are called the states of matter.

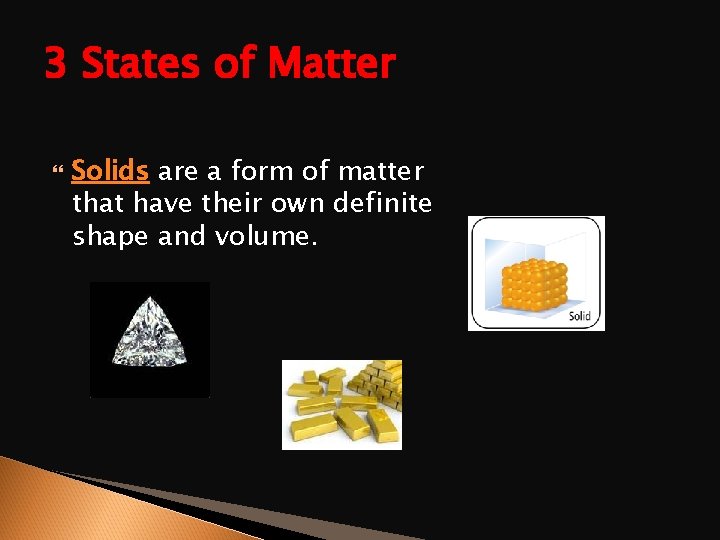
3 States of Matter Solids are a form of matter that have their own definite shape and volume.

3 States of Matter Liquids are a form of matter that flow, has constant volume and takes the shape of its container.

Gases have no definite shape or volume. They expand to fill their container. Like liquids gases also flow.

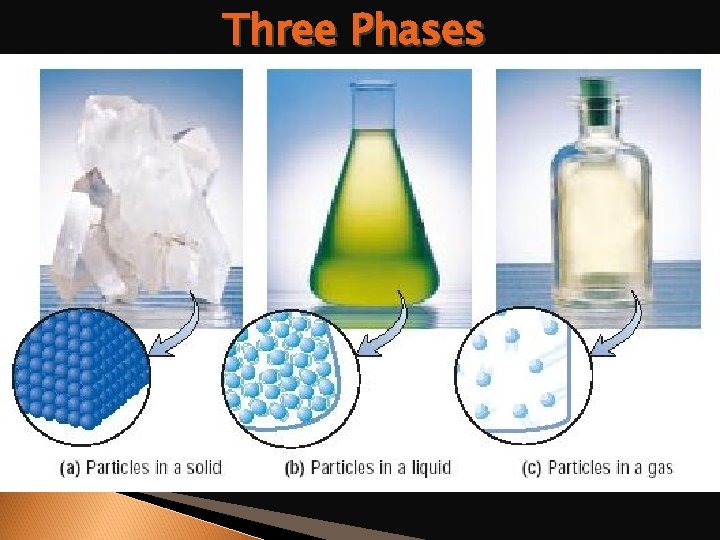
Phase Differences Solid – definite volume and shape; particles packed in fixed positions. Liquid – definite volume but indefinite shape; particles close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Plasma – high temperature, ionized phase of matter as found on the sun.

Three Phases

Copper Phases - Solid

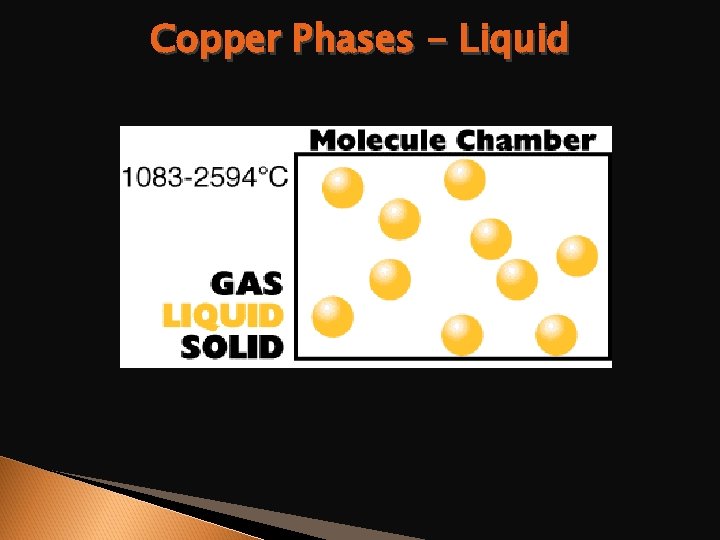
Copper Phases - Liquid

Copper Phases – Vapor (gas)

Solid, Gas & Liquid

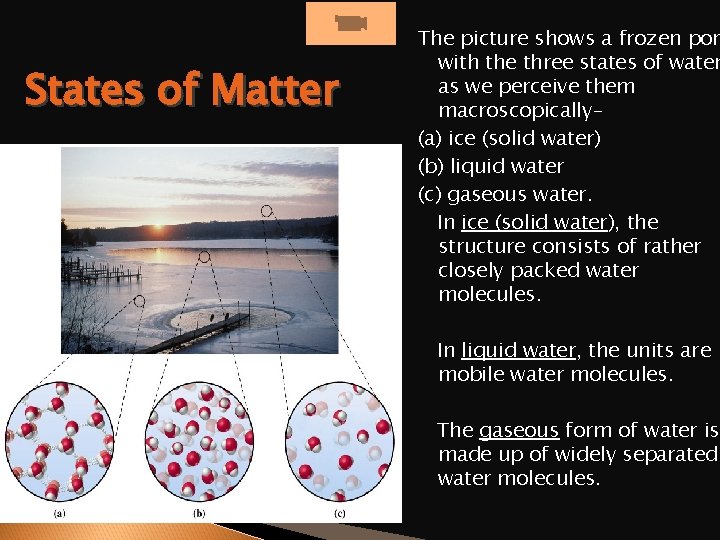
States of Matter The picture shows a frozen pon with the three states of water as we perceive them macroscopically– (a) ice (solid water) (b) liquid water (c) gaseous water. In ice (solid water), the structure consists of rather closely packed water molecules. In liquid water, the units are mobile water molecules. The gaseous form of water is made up of widely separated water molecules.

Review Classify each as a homogeneous or heterogeneous mixture Salad dressing Dirt Black coffee Air Paint Beach sand Spaghetti sauce Tap water

Review What are the 3 types of homogeneous solutions?

Schedule of Events Ø Today ◦ ◦ ◦ Free write – Part one Demonstration and Discussion Physical vs. Chemical Notes Physical vs. Chemical Properties/Changes Practice Go over WS Preview lab and begin pre-lab ØTomorrow ◦ ◦ Finish pre-lab Complete lab Revisit Demonstration Free write – Part two

Learning Targets – October 1 -2, 2013 I can distinguish between physical and chemical properties. I can describe the differences between physical and chemical changes using the correct terminology. I can name the six phase changes and describe the states of matter involved. I can describe what occurs during a splint test including what gases are present based upon the outcome of the test.

Free Write – Part One Take a full sheet of notebook paper and fold it in half. Above the crease, on the top half of the paper, you have 3 minutes to write every thing you know about physical and chemical changes. Write whatever comes to mind, doesn’t matter if it ends up being wrong later. Try to remember anything you have learned in the past.

ELEPHANT TOOTHPASTE DEMO

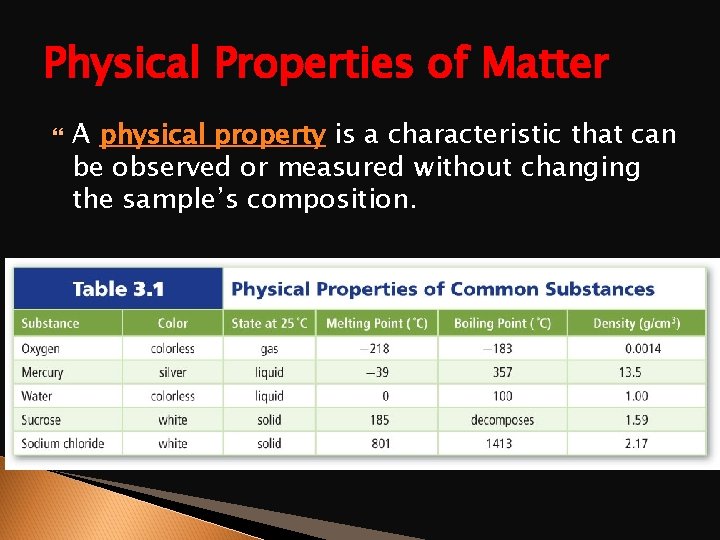
Physical Properties of Matter A physical property is a characteristic that can be observed or measured without changing the sample’s composition.

Chemical Properties of Matter The ability of a substance to combine with or change into one or more other substances is called a chemical property.

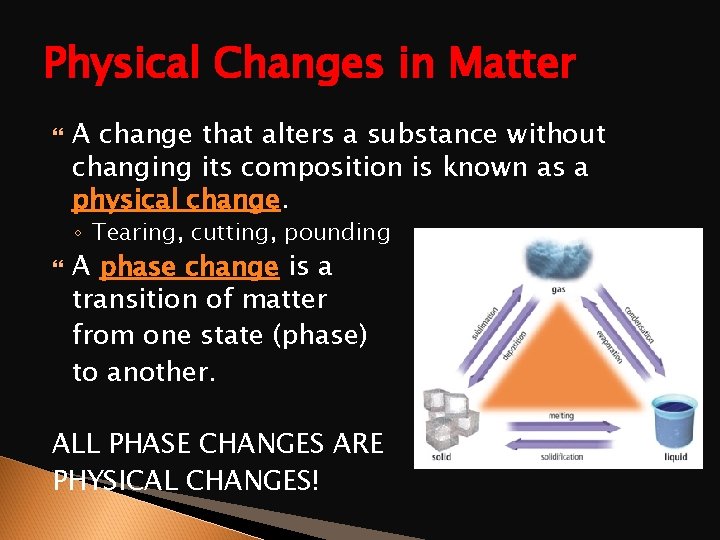
Physical Changes in Matter A change that alters a substance without changing its composition is known as a physical change. ◦ Tearing, cutting, pounding A phase change is a transition of matter from one state (phase) to another. ALL PHASE CHANGES ARE PHYSICAL CHANGES!

The Six Phase Changes ENERGY ADDED Solid Liquid = Melting Liquid Gas = Evaporation (Vaporization) Solid Gas = Sublimation ENERGY GIVEN OFF Liquid Solid = Freezing Gas Liquid = Condensation Gas Solid = Deposition

Chemical Changes in Matter A change that involves one or more substances turning into new substances is called a chemical change. Decomposing, rusting, exploding, burning, or oxidizing are all terms that describe chemical changes.

Chemical Changes in Matter Evidence of a chemical change: ◦ Color change ◦ Formation of bubbles (gas given off) ◦ A solid is formed (precipitate) ◦ Energy given off in the form of heat, light or sound.

Splint Tests in the Laboratory Within the laboratory, when a gas is produced, we can test its composition by performing a (flaming) splint test. In this test, we light a splint on fire and put it near the end of the glassware (sometimes slightly into the vessel) where the reaction is occurring. By the reaction of the flame, we can determine what gas is present: Carbon Dioxide (CO 2) – The flame will go out Oxygen (O 2) – The flame will stay lit or get bigger Hydrogen (H 2) – There will be an audible “popping” noise

Physical or Chemical Change? Are the following physical or chemical changes? Tearing a piece of paper in half Burning a piece of paper A bicycle rusting Frying an egg Freezing chocolate covered bananas Bleaching your hair Leaves changing color

Physical vs. Chemical Worksheets In your packet, you are to complete the worksheets titled “physical vs. chemical properties” and “physical vs. chemical changes” – I will be walking around the room to help with any problems you may have. Use your notes and the knowledge you just gained to help guide you. We will go over the answers together in approximately 7 -10 minutes, so work diligently.

Free Write – Part two On the bottom half of the same sheet, you now have 3 more minutes to write what you know about physical and chemical changes now. If some things are the same as before, please write them down a second time. Anything new that you’ve learned, please write that down as well.

Conservation of Mass The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, it is conserved. massreactants = massproducts

Conservation of Mass massreactants = massproducts When sodium metal is added to chlorine gas, sodium chloride or table salt is formed. How much sodium chloride is formed when 5 g of sodium is added to 8 grams of chlorine gas?

How Do We Separate Mixtures? Filtration is a technique that uses a porous barrier to separate a solid from a liquid in a heterogeneous mixture.

How Do We Separate Mixtures? Distillation is a separation technique for homogeneous mixtures that is based on the differences in boiling points of substances.

Separation of Mixtures Crystallization is a separation technique for homogenous mixtures that results in the formation of pure solid particles from a solution containing the dissolved substance.

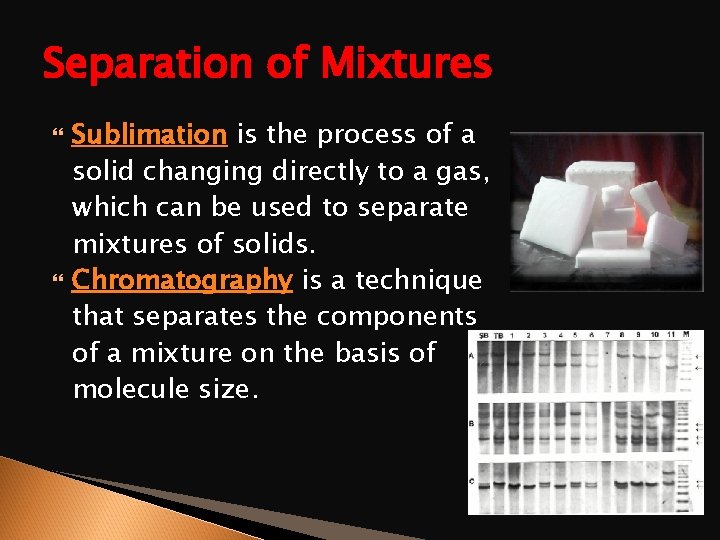
Separation of Mixtures Sublimation is the process of a solid changing directly to a gas, which can be used to separate mixtures of solids. Chromatography is a technique that separates the components of a mixture on the basis of molecule size.

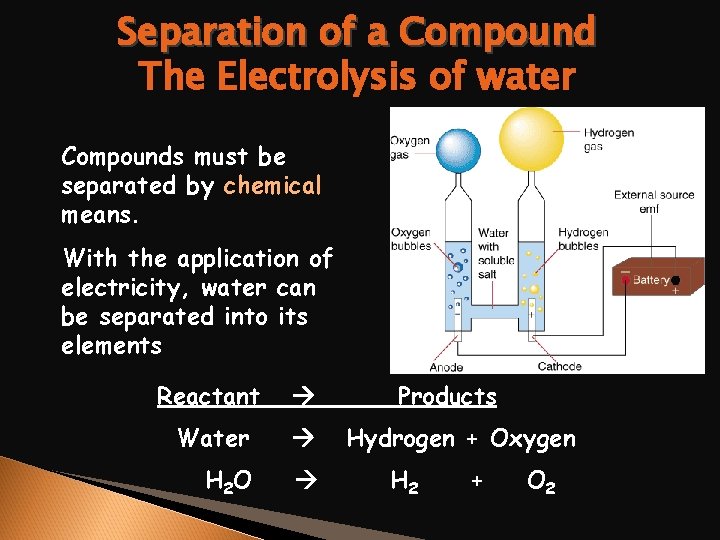
Separation of a Compound The Electrolysis of water Compounds must be separated by chemical means. With the application of electricity, water can be separated into its elements Reactant Water H 2 O Products Hydrogen + Oxygen H 2 + O 2

Wednesday, 10/5 Turn in Strange Matter worksheet Review ◦ ◦ ◦ Chemical vs. physical properties Chemical vs. physical changes Law of conservation of mass Heterogeneous vs. homogeneous mixtures 5 methods for separating mixtures. Element vs. compound Law of Definite Proportions ◦ Book problems

Review Chemical or physical property? ◦ ◦ ◦ Lead has a dull gray color. Lead combines with iodine to form yellow lead iodide. Aluminum has a density of 2. 7 g/cm 3. Aluminum reacts with acid (releasing hydrogen gas). Ethanol has a boiling point of 173 °F. Ethanol is flammable (will catch fire near an open flame).

Review Chemical vs. Physical change Liquid water when heated changes to steam. Sugar dissolving in water. Sugar heated in a sauce pan turns brown. Milk which has turned sour. A tire is inflated with air. Sodium metal when added to water will spark and release heat. ◦ A piece of sodium metal is cut in two. ◦ ◦ ◦

Review Law of conservation of mass ◦ A 10 g sample of mercury metal reacts with 6. 6 g of oxygen. How much mercury oxide is produced? ◦ 15 g of mercury metal reacts with oxygen to produce 20 g of mercury oxide. How much oxygen was used?

Review Heterogeneous vs. Homogeneous Mixtures ◦ Raisin bran muffin ◦ Pink lemonade ◦ Penny ◦ Cole slaw ◦ Cell phone What are the 5 techniques used to separate mixtures? Element vs. Compound? ◦ Ca (calcium) ◦ Ca. O (calcium oxide ◦ O 2 (oxygen) ◦ Cu. O (copper oxide) ◦ He (helium) ◦ NH 3 (ammonia) ◦ Gold

Law of Definite Proportions New Learning Goal I understand how to calculate the % by mass of an element within a compound. • The law of definite proportions states that a compound is always composed of the same elements in the same proportion by mass, no matter how large or small the sample.

Law of Definite Proportions Water, H 2 O is composed of the elements oxygen, O and hydrogen, H. ◦ The amount by mass of hydrogen in water is always in the same proportion or percentage, 11% REGARDLESS of the total quantity of water present. ◦ The amount by mass of oxygen in water is always in the same proportion or percentage, 89% REGARDLESS of the total quantity of water present. Example ◦ If I have an 18 g sample of water, H 2 O, what is the mass of hydrogen and oxygen present?

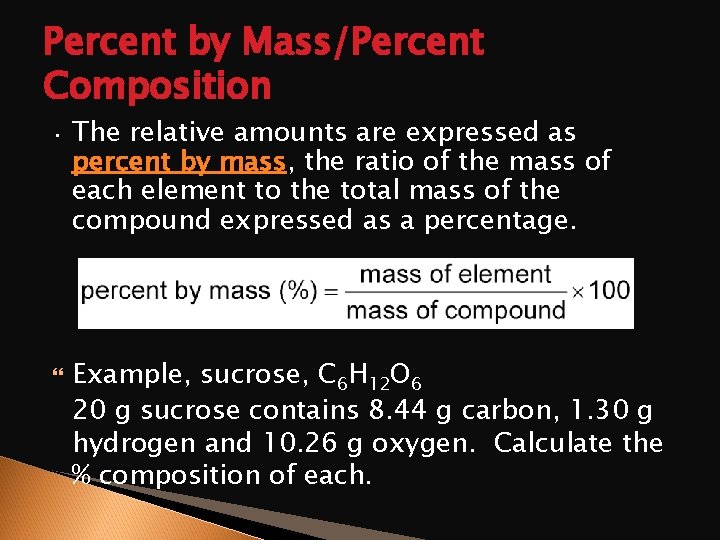
Percent by Mass/Percent Composition • The relative amounts are expressed as percent by mass, the ratio of the mass of each element to the total mass of the compound expressed as a percentage. Example, sucrose, C 6 H 12 O 6 20 g sucrose contains 8. 44 g carbon, 1. 30 g hydrogen and 10. 26 g oxygen. Calculate the % composition of each.

Law of Multiple Proportions The law of multiple proportions states that when different compounds are formed by a combination of the same elements, different masses of one element combine with the same relative mass of the other element, in small, whole-number ratios. Example – both water and hydrogen peroxide only contain hydrogen and oxygen, but water contains a 2: 1 ratio of hydrogen to oxygen (H 2 O) while hydrogen peroxide contains a 2: 2 ratio of hydrogen to oxygen (H 2 O 2)