Matter Properties and Changes 1 Physical Properties can










- Slides: 13

Matter: Properties and Changes 1

Physical Properties can be observed without changing the identity of the substance. Ex… ~ co l o r ~ taste ~ odor ~ size ~ shape ~ texture ~ conductivity ~ viscosity ~ elasticity ~ hardness ~ magnetism ~ boiling/ melting point and many more… 2

But one of the most useful is Density: the amount of matter present in a given volume. ~ or how heavy a substance is compared to how much space it takes up Ice is less dense than water; it floats on water A is more dense than B (there is more matter and less space); B and C are of similar densities b/c the ratios of matter and space are about the same 3

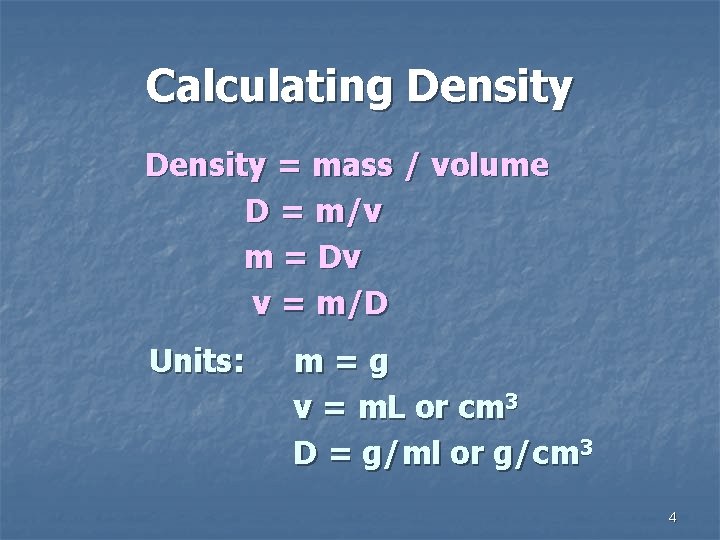
Calculating Density = mass / volume D = m/v m = Dv v = m/D Units: m=g v = m. L or cm 3 D = g/ml or g/cm 3 4

Density Problems 1. A nail has a vol. of 0. 880 cm 3 and a mass of 6. 920 grams. What is the density of the nail? D = m/v D = 6. 920 g ÷ 0. 880 cm 3 D = 7. 86 g/cm 3 2. Vegetable oil has a density of 0. 916 g/ml. Calculate the mass of 500. 0 ml of oil. m=D×v m = 0. 916 g/ml × 500. 0 ml m = 458 grams 3. The density of a piece of wood is 0. 86 g/cm 3. What is the volume of the wood if its mass is 75 grams? v = m/D v = 75 g ÷ 0. 86 g/cm 3 v = 87 cm 3 5

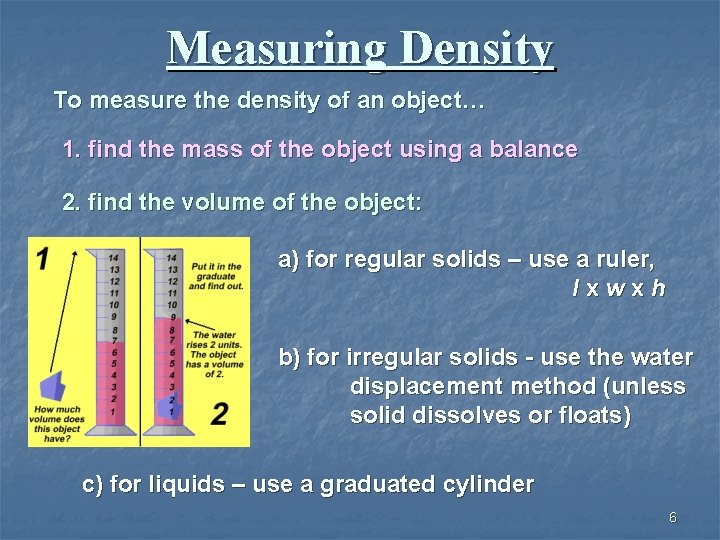
Measuring Density To measure the density of an object… 1. find the mass of the object using a balance 2. find the volume of the object: a) for regular solids – use a ruler, lxwxh b) for irregular solids - use the water displacement method (unless solid dissolves or floats) c) for liquids – use a graduated cylinder 6

Buoyancy - Density and buoyancy are physical properties - Buoyancy is the force with which a more dense fluid pushes a less dense substance upward 7

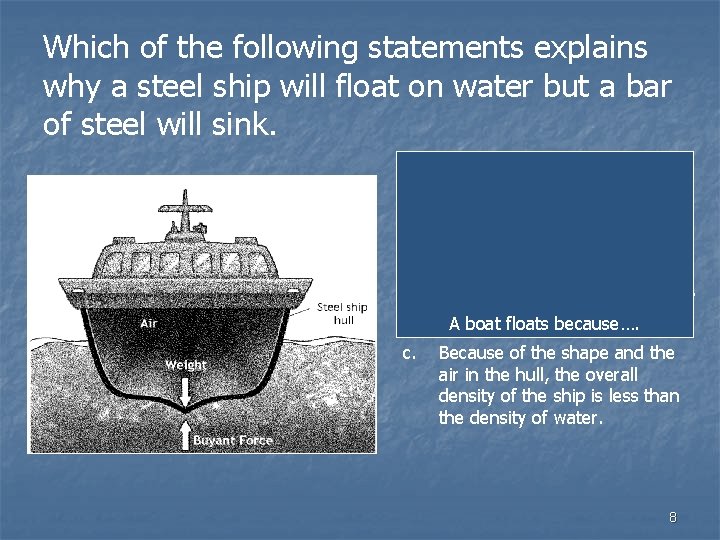
Which of the following statements explains why a steel ship will float on water but a bar of steel will sink. a. When rolled into a sheet, steel is less dense than water. b. The densest part of the ship is the part that is out of the water. A boat floats because…. c. Because of the shape and the air in the hull, the overall density of the ship is less than the density of water. 8

Physical Changes are changes in appearance only… NOT in chemical make-up Includes… ~ changes in size ~ changes in shape ~ changes in phase ~ dissolving 9

Examples of physical changes. . . ~ breaking a window ~ melting a piece of ice ~ tearing / cutting a piece of paper ~ dissolving sugar in hot tea ~ painting wood ~ shaping metal 10

Chemical Properties properties that explain HOW a substance will react or behave. The substance must be changed into something new to observe the property. Ex: ~ flammability ~ reactivity to… water acids bases ~ ability to lose electrons (be oxidized) 11

Chemical Changes Are changes that result in a recombination of atoms – the formation of NEW substance(s)! ~ you can’t get the original substance back without a chemical reaction. Evidence that a chemical change has occurred: ~ formation of precipitate (solid) ~ gas / bubbles given off ~ light given off ~ color change (if new substance) ~ heat given off / absorbed (breaking/making chem. bonds) 12

Examples of chemical changes: Striking a match releases heat and light and results in ash, smoke and gas ~ burning a match ~ silver tarnishing Cooking bread changes the color, texture, odor, taste ~ baking bread ~ food rotting or souring ~ a nail rusting 13