Matter Matter is anything that occupies space and




- Slides: 10

Matter

Matter is anything that occupies space and has mass. n Everything around you is matter – you, the chairs – even the air is matter (even though you can’t see it!) n Light and sound are not matter – they have no mass or volume n

Atoms Smallest particle that maintains the properties of the element. n Are atoms indivisible? n Can you see an atom with your naked eye? n No, but if we could, it would look like this n We CAN see groups of atoms n

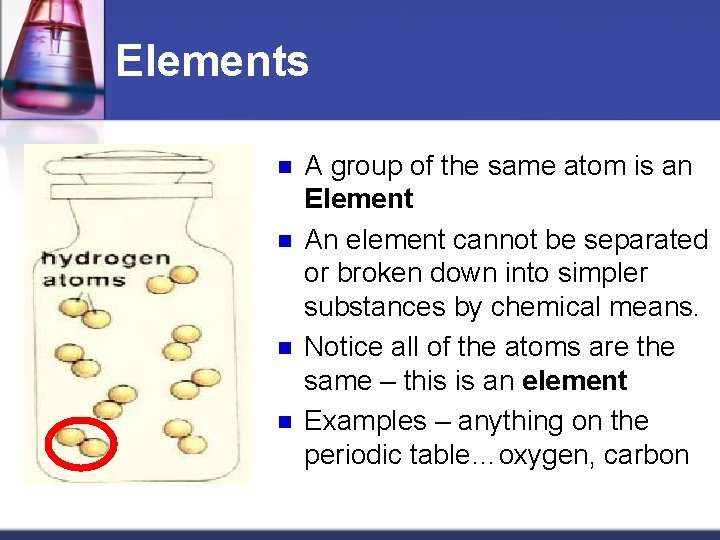
Elements n n A group of the same atom is an Element An element cannot be separated or broken down into simpler substances by chemical means. Notice all of the atoms are the same – this is an element Examples – anything on the periodic table…oxygen, carbon

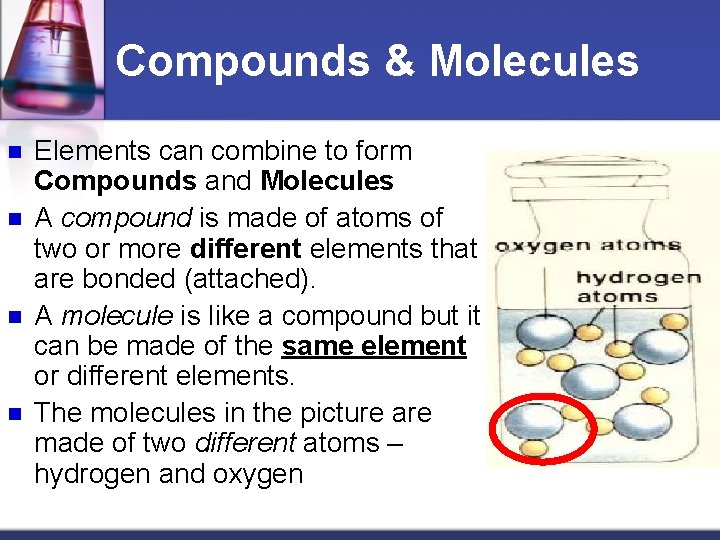
Compounds & Molecules n n Elements can combine to form Compounds and Molecules A compound is made of atoms of two or more different elements that are bonded (attached). A molecule is like a compound but it can be made of the same element or different elements. The molecules in the picture are made of two different atoms – hydrogen and oxygen

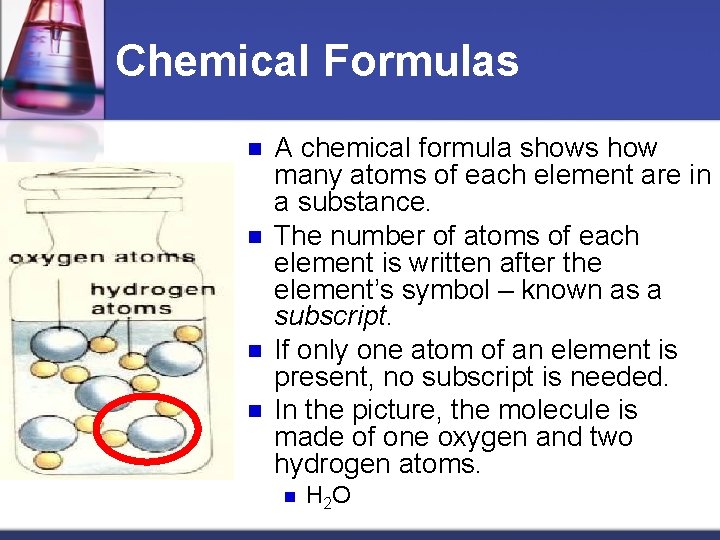
Chemical Formulas n n A chemical formula shows how many atoms of each element are in a substance. The number of atoms of each element is written after the element’s symbol – known as a subscript. If only one atom of an element is present, no subscript is needed. In the picture, the molecule is made of one oxygen and two hydrogen atoms. n H 2 O

Pure Substances n. A single element or a single compound – has a definite chemical formula n Water has the formula H 2 O n Salt has the formula Na. Cl

Mixtures n n Combination of two or more substances that are NOT chemically combined. These substances CAN be separated from each other Here is what a mixture could look like on the atomic level This is a mixture of hydrogen and oxygen (notice the hydrogen and oxygen are NOT bonded together)

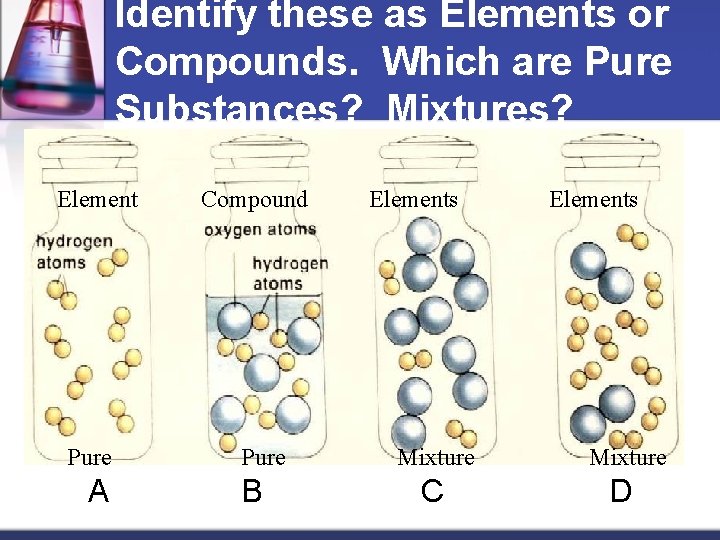
Identify these as Elements or Compounds. Which are Pure Substances? Mixtures? Element Pure A Compound Pure B Elements Mixture C Elements Mixture D

Can You Separate a Mixture? Time for a Lab!!