MATTER Matter is anything that has mass and


















































































- Slides: 118

MATTER


Matter is anything that has mass and takes up space.

Mass is a measure of the amount of matter in an object.

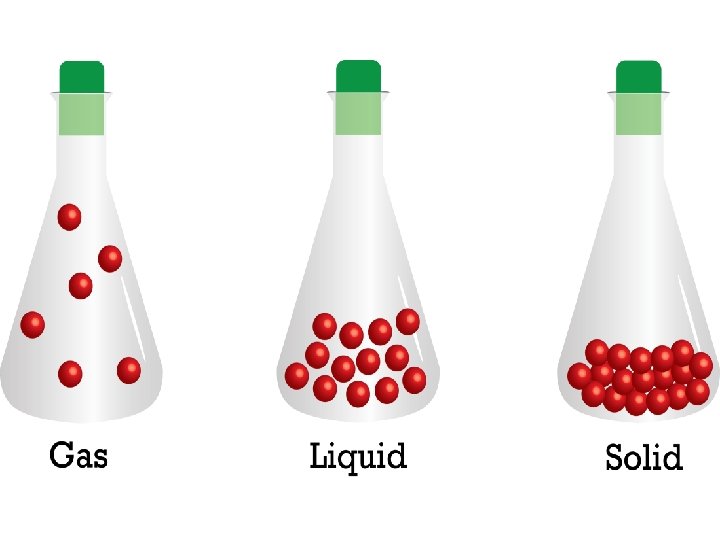
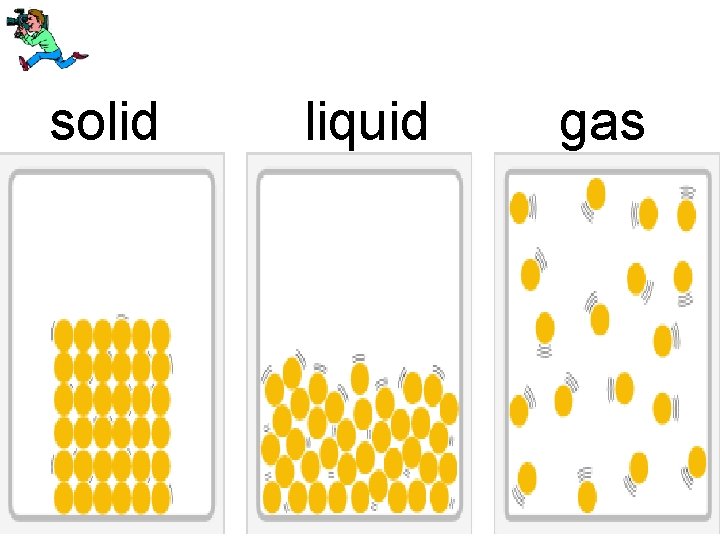
4 forms of matter • solid • liquid • gas • plasma


solid liquid gas

SOLIDS

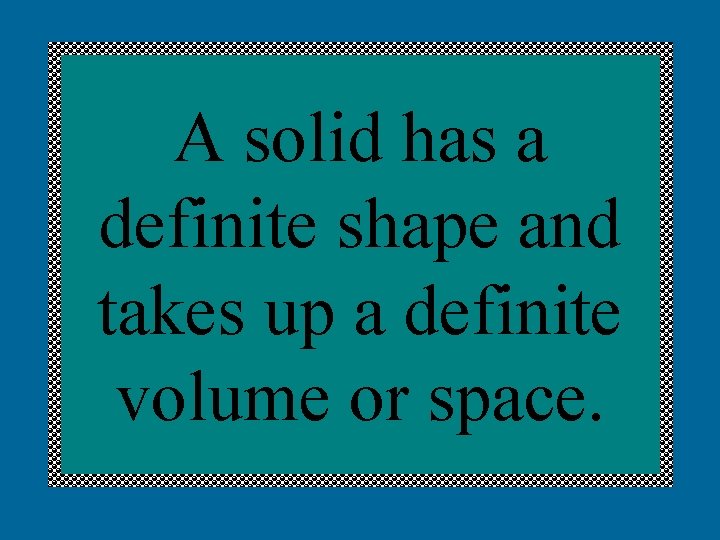
A solid has a definite shape and takes up a definite volume or space.

In a solid the particles vibrate but do not move around.



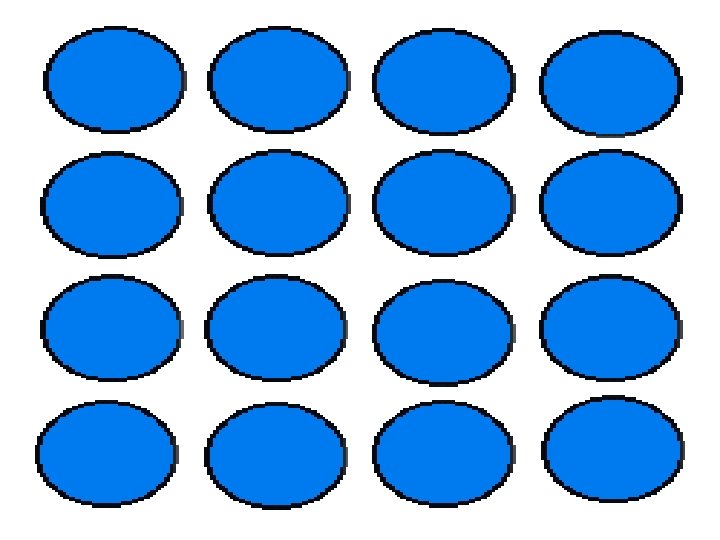
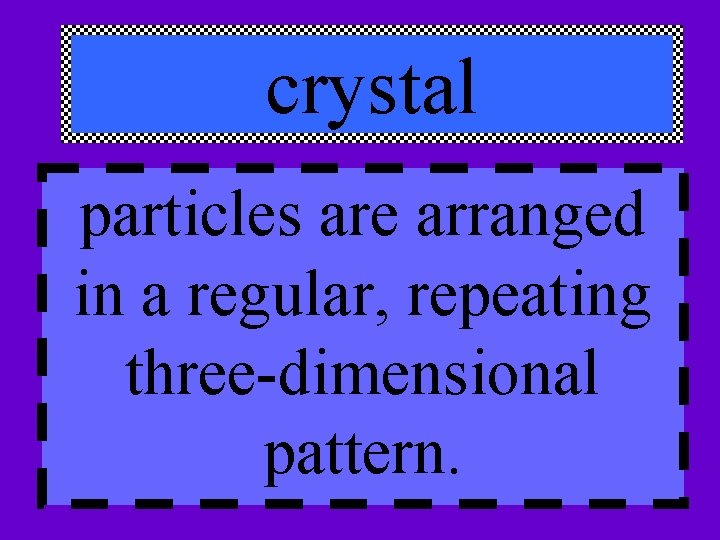
2 groups of solids • crystal • amorphous

crystal particles are arranged in a regular, repeating three-dimensional pattern.

This organized structure is called a crystal lattice, which is created when a solid of pure elements or compounds freezes.

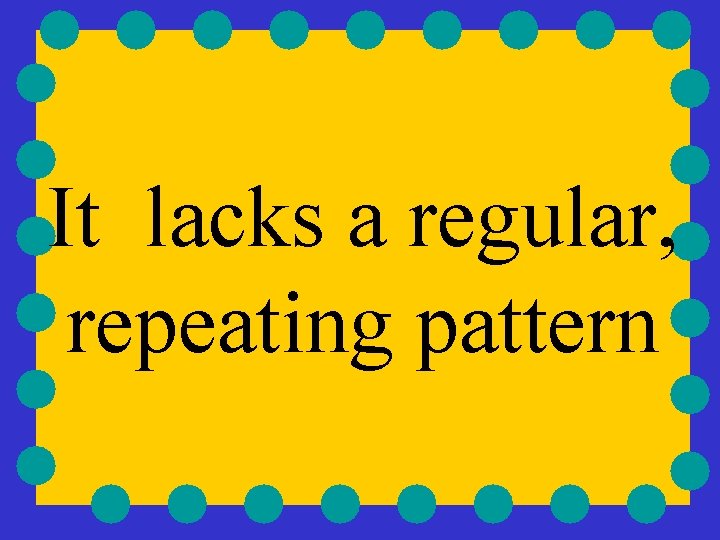
amorphous has particles which are jumbled together

It lacks a regular, repeating pattern

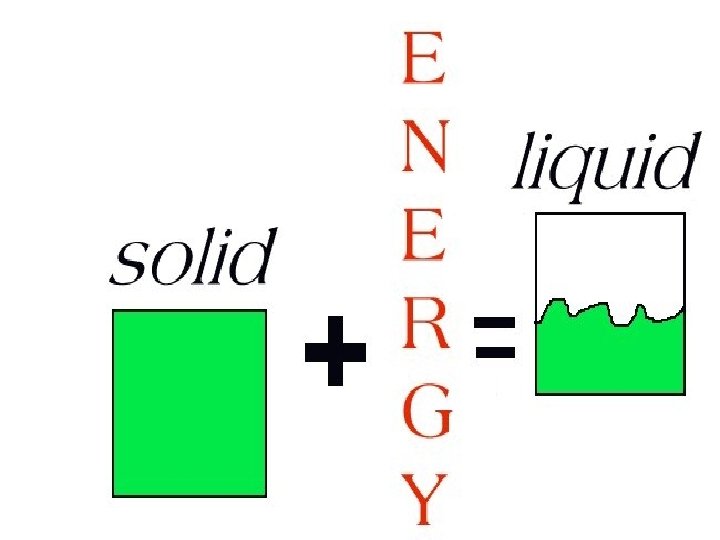
To change a solid into a liquid, a form of energy must be added to have the atoms move faster.


LIQUIDS

Liquids have a definite volume but not a definite shape.

Forces between the particles in liquids are farther apart and not strong enough to hold a liquid in any definite shape.



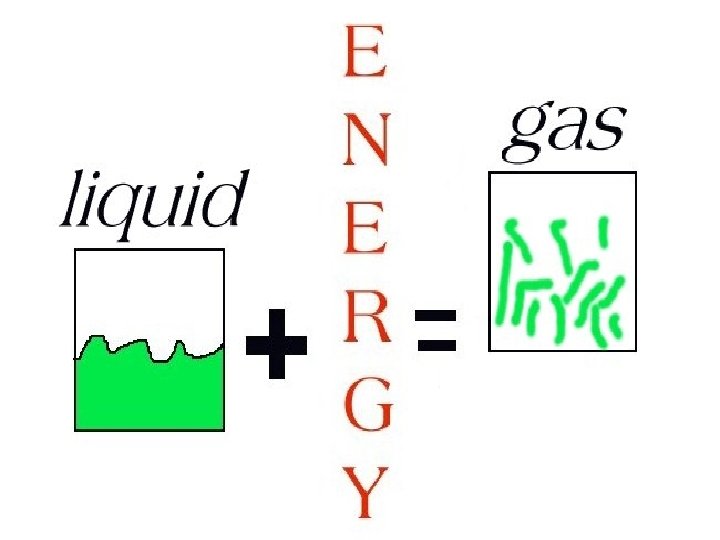
To change a liquid into a gas, an energy source must be added.


GAS

A gas does not have a definite shape or volume.

Gas particles are very far apart compared to those of a liquid or a solid.

The forces between the gas particles are very weak.

Gas particles move quickly in straight lines, changing direction when they collide with each other or hit the walls of a container.



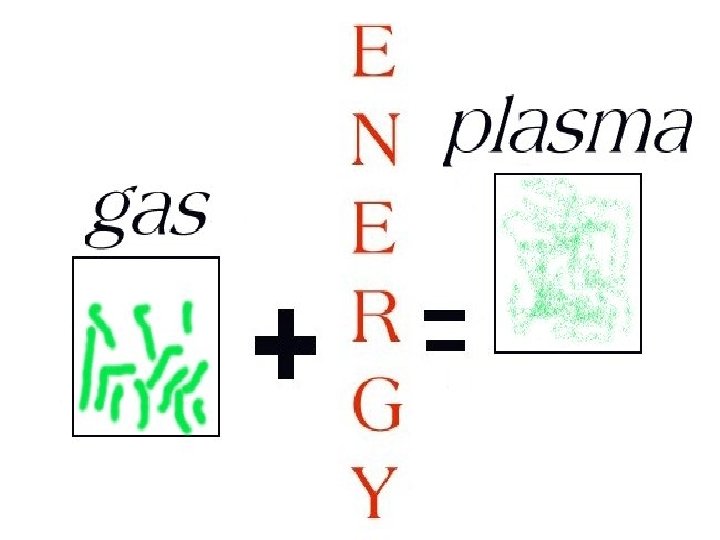
A gas can be subjected to an energy source and changed into a plasma.


PLASMA

The sun and other stars are in a plasma state.

Plasma particles shake violently at very high temperatures and are electrically charged.

Substances can be changed from one state to another.

Particles are held together by forces. These forces give a solid its definite shape.

PHYSICAL PROPERTIES OF MATTER

Physical properties are characteristics of matter that can be studied without changing the make-up of a substance.

Color, taste, odor, and melting temperature are physical properties.

The physical properties of a kind of matter stay the same regardless of the shape or amount of that matter.

viscocity a liquid’s resistance to flow

Water has a LOW viscocity and flows EASILY. Motor oil has a HIGH viscocity and flows SLOWLY.

Surface Tension

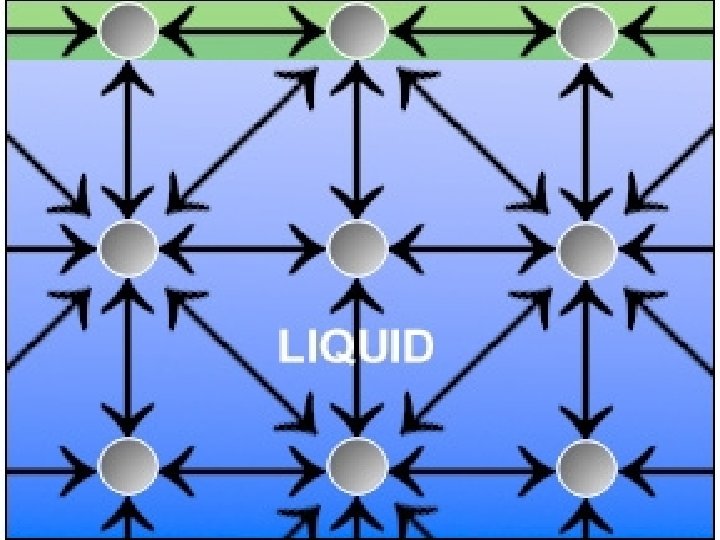
Surface tension is the tendency of a liquid to form a skin at the surface as the particles move closer together.


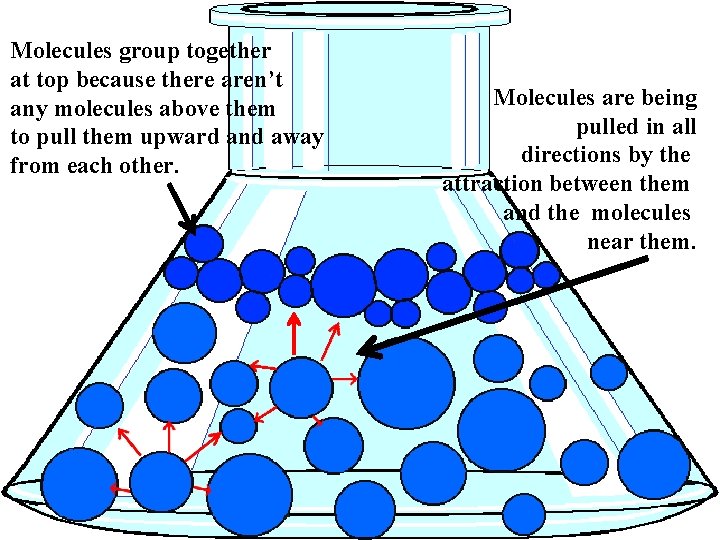
Molecules group together at top because there aren’t any molecules above them to pull them upward and away from each other. Molecules are being pulled in all directions by the attraction between them and the molecules near them.





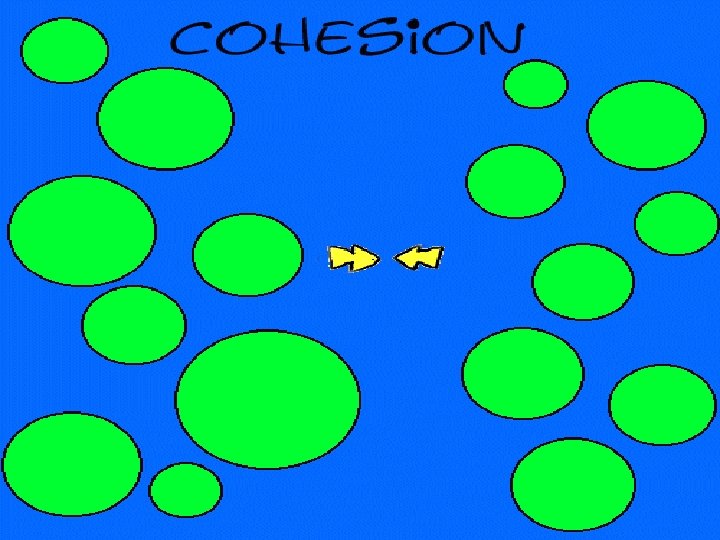
cohesion moving together of particles of the same substance


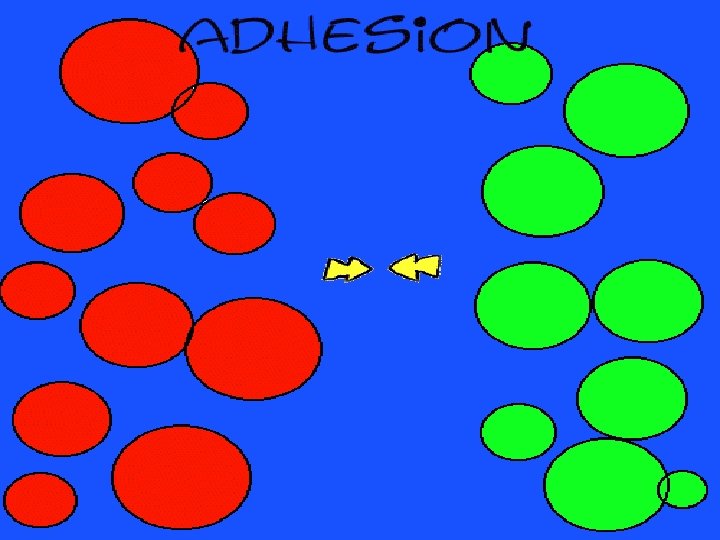
adhesion moving together of particles of different substances


PROPERTIES OF SOLIDS

elasticity quality of a solid to be stretched and then return to its original shape


malleable solids can be hammered into thin sheets


brittleness break when hammered


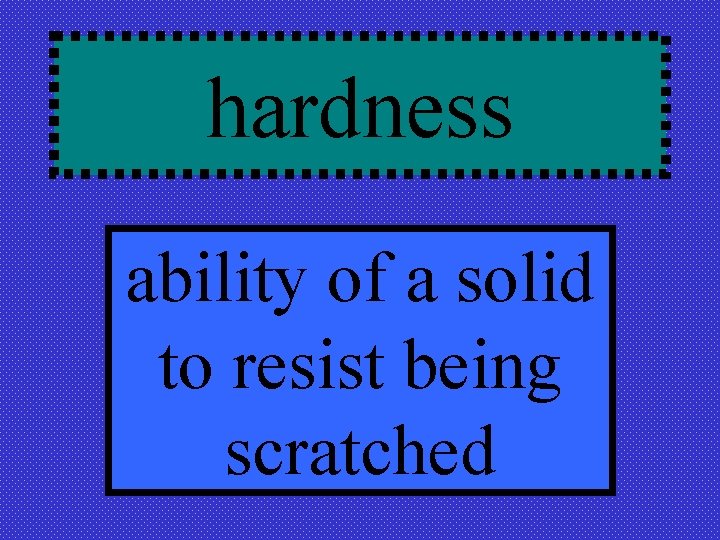
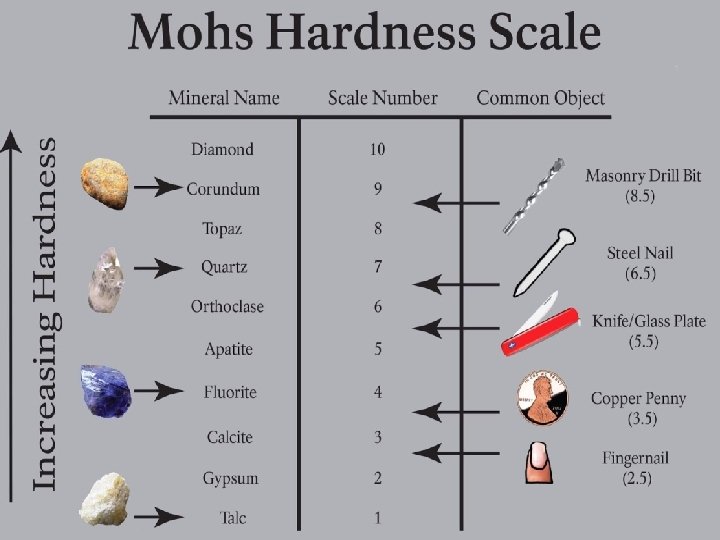
hardness ability of a solid to resist being scratched


ductility ability of a solid to be drawn into a wire


tensile strength how well a solid resists breaking under tension


Knowledge of the physical properties help people choose the best ways to use different types of matter.

CHANGES OF STATE

The temperature at which changes take place are physical changes.

melting point temperature at which a solid becomes a liquid

freezing point temperature which a liquid returns to or becomes a solid

The point at which a substance melts or freezes depends on how strong the forces are that attract their particles.

A liquid can change to a gas state by evaporation or by boiling.

Evaporation takes place at the surface of a liquid.

Boiling takes place all through a liquid.

The boiling point is the temperature at which a liquid boils at sea level.

Condensation is the reverse of boiling.

In evaporation, a liquid turns into a gas. In condensation, a gas (water vapor) turns into a liquid.

The temperature at which a gas condenses and changes to a liquid is called the condensation point.

A physical change is one in which the appearance of matter changes, but its chemical properties remain the same.

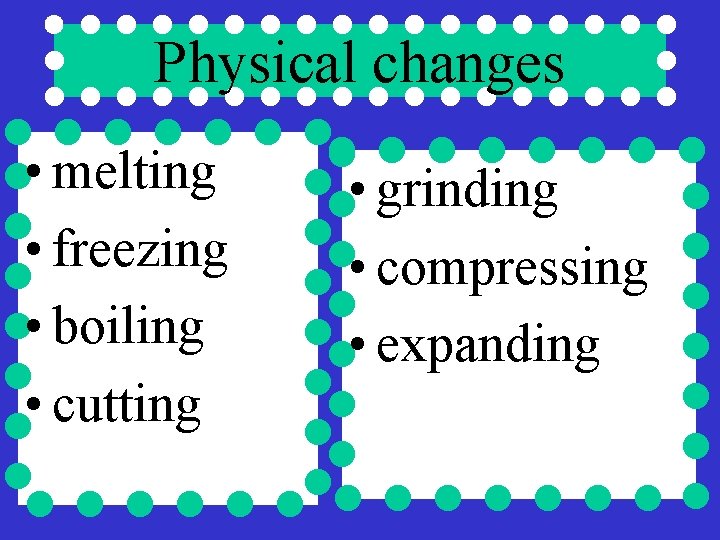
Physical changes • melting • freezing • boiling • cutting • grinding • compressing • expanding


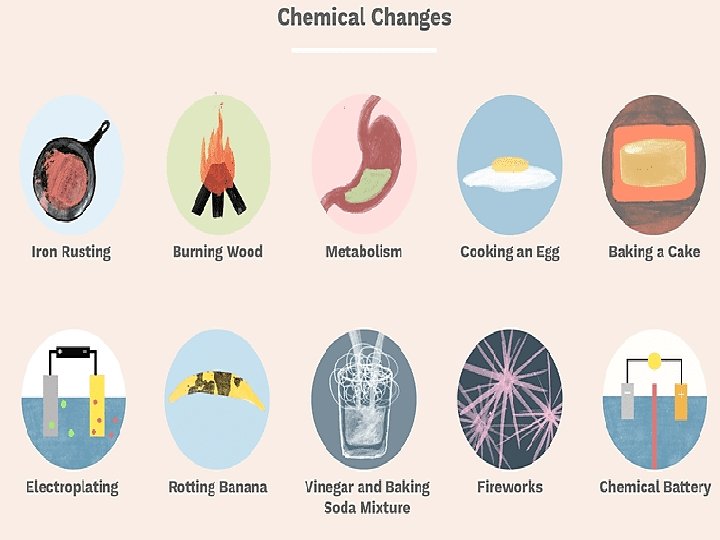
Burning causes chemical changes in matter.

A chemical change is a change that produces one or more kinds of matter different from those present before the change.


Mixtures, Solutions, and Compounds

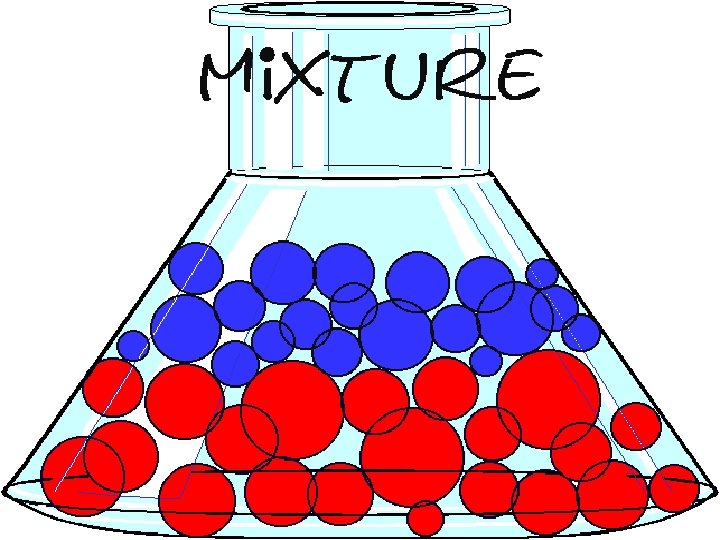
A mixture is formed when two or more substances that can be physically separated is produced.

A mixture has a definite line separating the layers of the different substances.




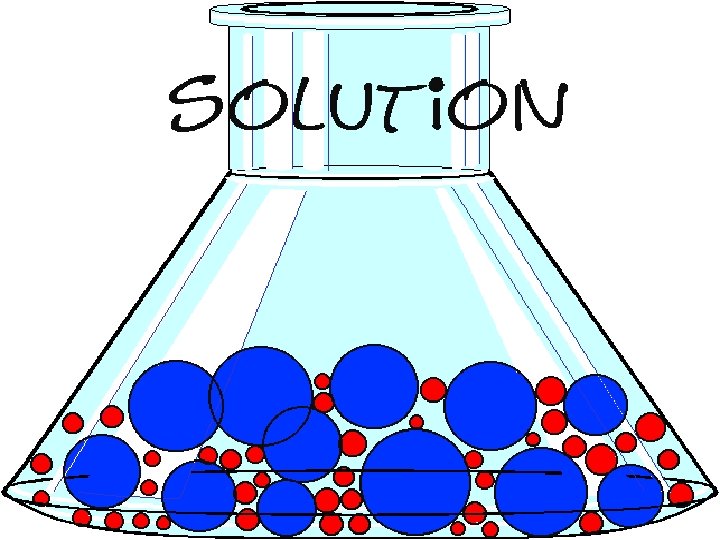
A solution is made when one or more liquid, gaseous, or solid substance is dispersed in another.

A solution has small pieces of one substance intermixed with pieces of another substance.


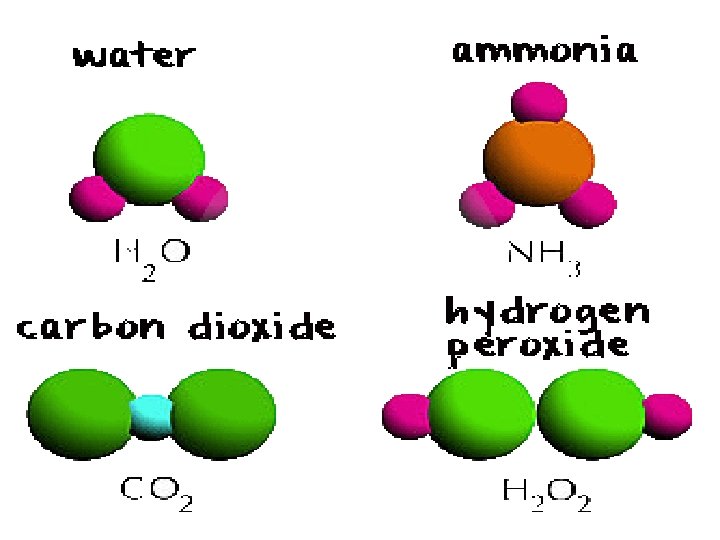
A compound exists when 2 or more elements are chemically bonded together.

A compound joins together molecules of more than one element to create a new substance.


EXAMPLES

MIXTURE • vinegar/ water • oil/ water • sand/water

SOLUTION • sugar/ water • drink mix/water

COMPOUND • Iron/ oxygen (iron oxide, rust) • sodium/ chloride (salt) • hydrogen/ oxygen (water)

Mixture, Solution, Compound Examples







Chemical reactions can cause a change in temperature.

Exothermic reactions transfer energy to the surroundings making it get hotter.

An example would be a hand warmer. The chemical reaction creates heat which warms the area.

Endothermic reactions take in energy making the surroundings colder.

An example would be an instant ice pack. The chemical reaction cools the surrounding area.