Matter Its what the worlds made of Matter













- Slides: 26

Matter: It’s what the world’s made of.

Matter is anything that has mass and volume. Matter can be solid, liquid or gas or combination of these states. For example, foam is a mixture of a liquid and a gas, or a solid and a gas. MATTER n Anything that has mass and volume n can be solid, liquid or gas (or a combo of…)
Matter Mass is a measure of the quantity of matter in an object. Mass is often measured in kilograms (kg) or in grams (g). MASS n Measure of the quantity of matter in an object n Measured in ‘kg’ or ‘g’

Matter Volume is a measure of how big an object is or how much space a fluid takes up. Volume is often measured in litres (L) or in millilitres (m. L). VOLUME n Measure of how much space an object or fluid takes up n Measured in ‘L’ or ‘m. L’

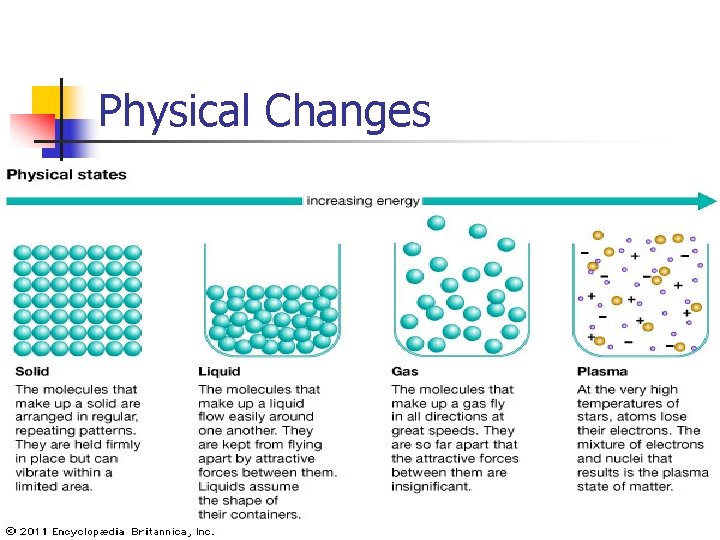
The Particle Theory of Matter All matter is made of tiny particles. Different kinds of matter are made of different kinds of particles. For example, the particles that make up water are different from the particles that make up the glass containing it. The Particle Theory of Matter is a way to describe the structure of matter and its behaviour. PARTICLE THEORY OF MATTER n Theory that describes the composition and behaviour of matter

The Particle Theory of Matter According to The Particle Theory: n Particles are attracted to each other and are always moving n When heated, particles gain energy and begin to move faster n The distances between the particles change for different states of matter n The amount of attraction is different for different kinds of particles

The Particle Theory of Matter 1. 2. 3. 4. 5. All matter is made up of tiny particles. All particles have empty spaces between them Different substances are made up of different kinds of particles Particles are in motion and move faster as temperature increases Particles attract each other.

What do you know about matter? Solids Gasses Liquids Plasma

Solids n n n Solids hold their own shape. Solids have weight. Solids take up space. Read more!

Liquids n n n Liquids take the shape of their container. Liquids have weight. Liquids take up space. Read more!

Gases n n n Gases spread out to fill the entire space given. Gases have weight. Gases take up space. Read more!

Plasma n n n Lightning is a plasma. Used in fluorescent light bulbs and Neon lights. Plasma is a lot like a gas, but the particles are electrically charged. Read more!

STATES of matter? What would it take for matter to move from one state to another?

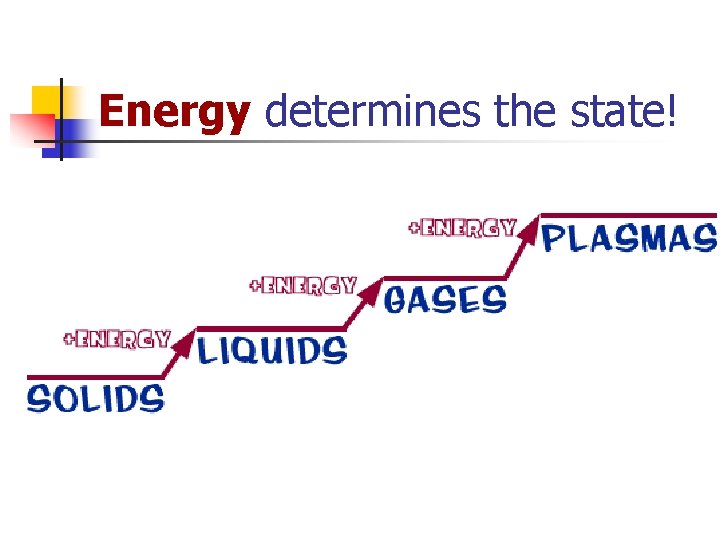
Energy determines the state!

Particles in Solids: n n Are packed tightly together Have very little energy

Particles in Liquids: n n Are loosely packed Have medium energy levels

Particles in Gases: n n Move freely Have LOTS of energy

Particles in Plasma: n n Are electrically charged Have EXTREMELY high energy levels

Physical Changes

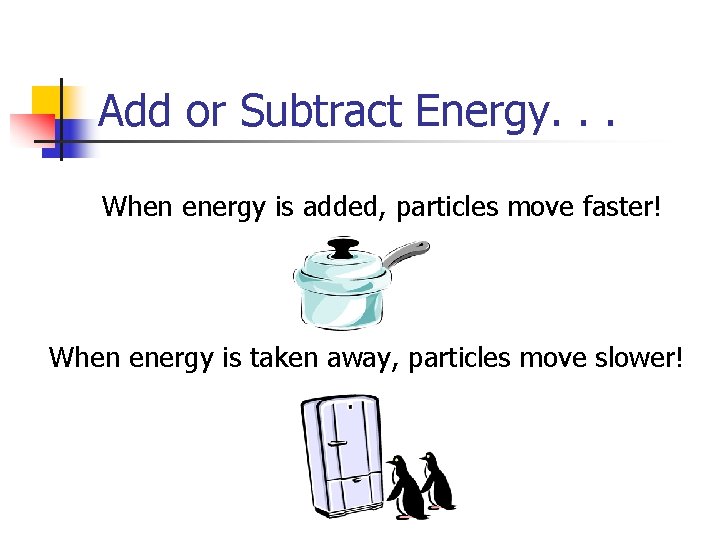
Add or Subtract Energy. . . When energy is added, particles move faster! When energy is taken away, particles move slower!

What will happen? Why?

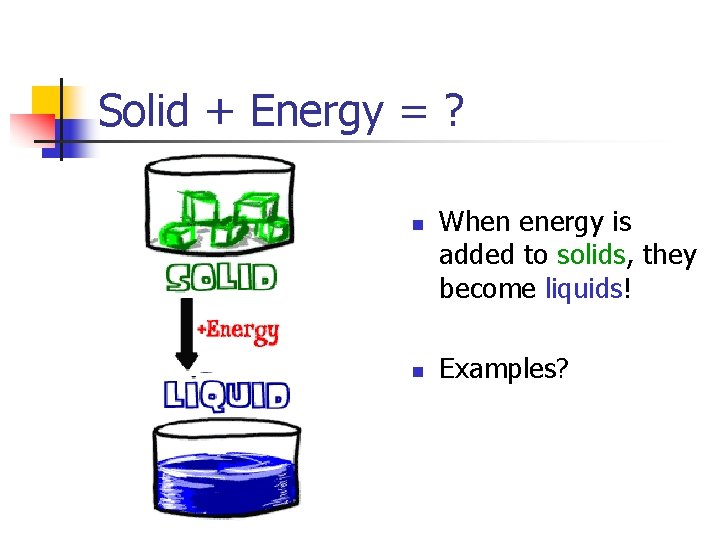
Solid + Energy = ? n n When energy is added to solids, they become liquids! Examples?

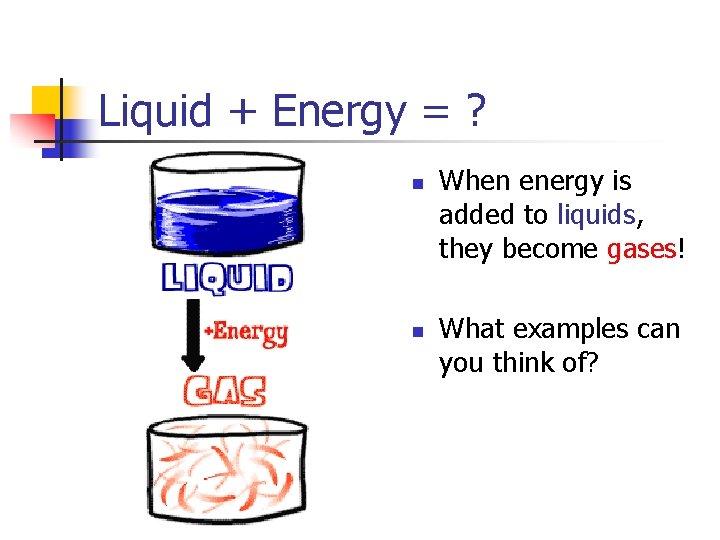
Liquid + Energy = ? n n When energy is added to liquids, they become gases! What examples can you think of?

Changing States n There are several names for matter changing states: n State change n Phase change n Physical change

So, did we get something new? n n Ice cream and melted ice cream? Chocolate and melted chocolate? Ice, water, and water vapor? Steel and molten steel? These changes are called: physical changes!

The End!