Matter is made up of particles which are











- Slides: 30


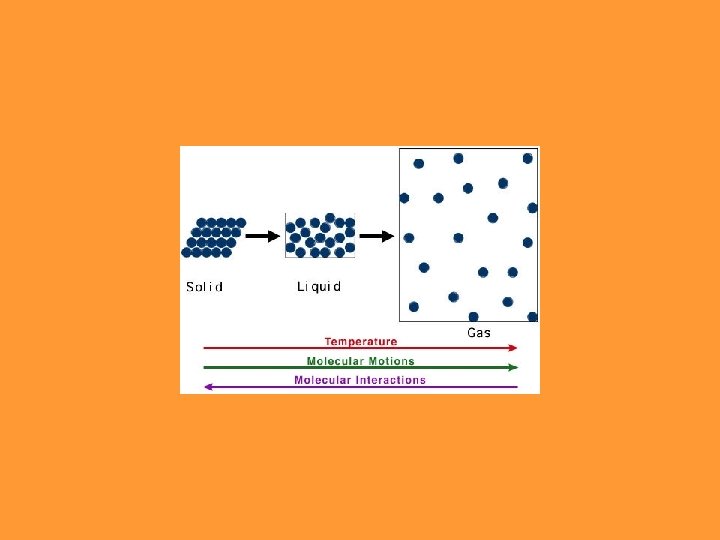
Matter is made up of particles which are in continual random motion.

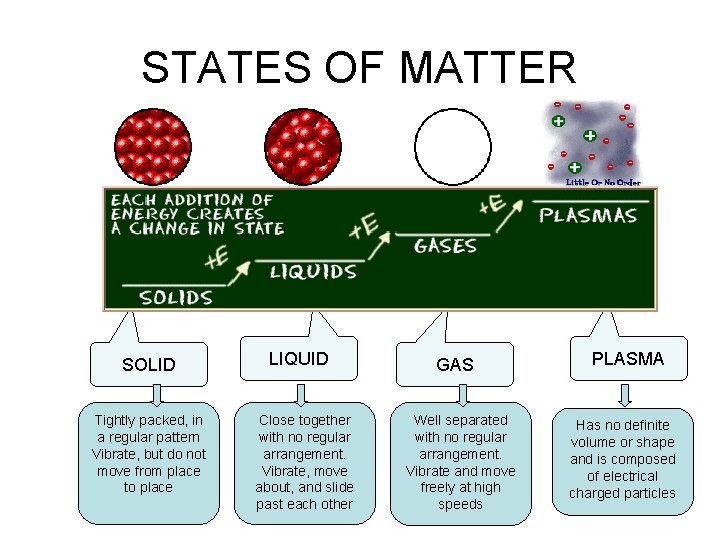
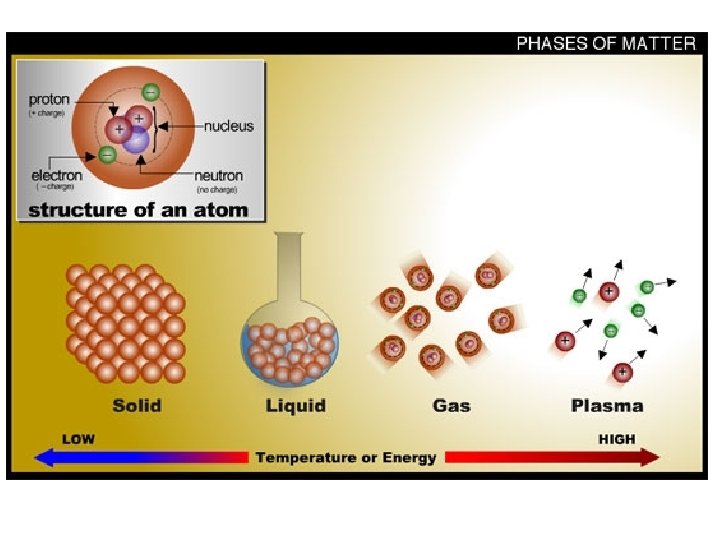
There are 4 States of Matter 1) Solid 2) Liquid 3) Gas 4) Plasma

THE STATE MATTER IS IN DEPENDS ON: ØBased upon particle arrangement ØBased upon energy of particles ØBased upon distance between particles

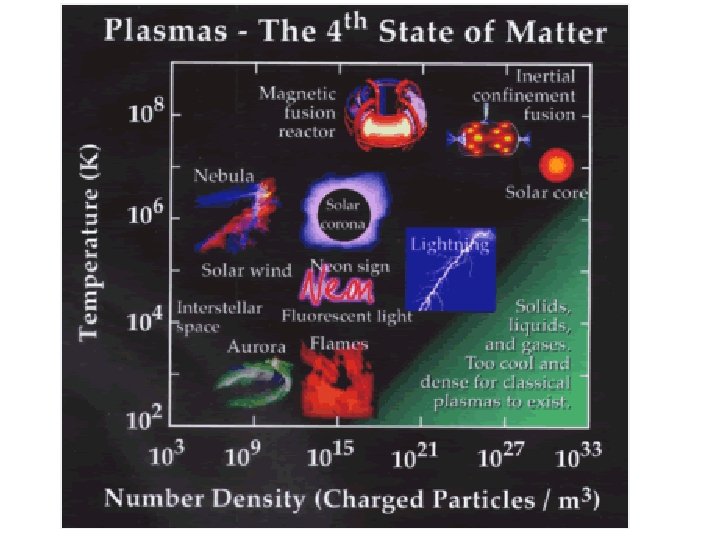
(d) plasma

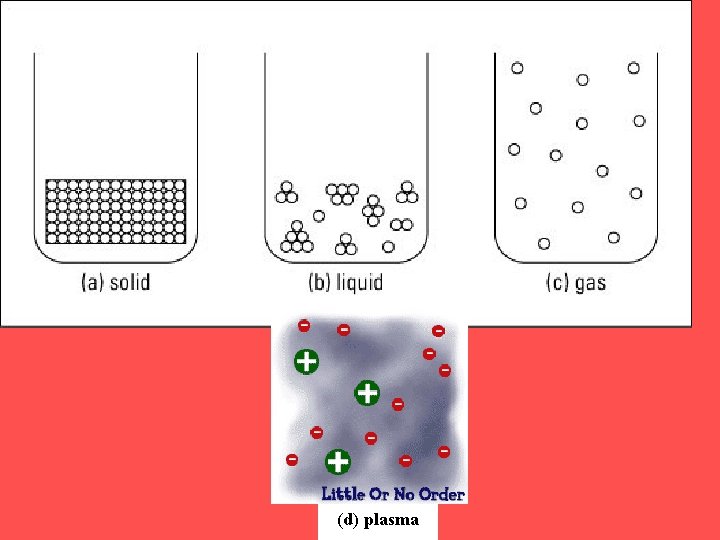
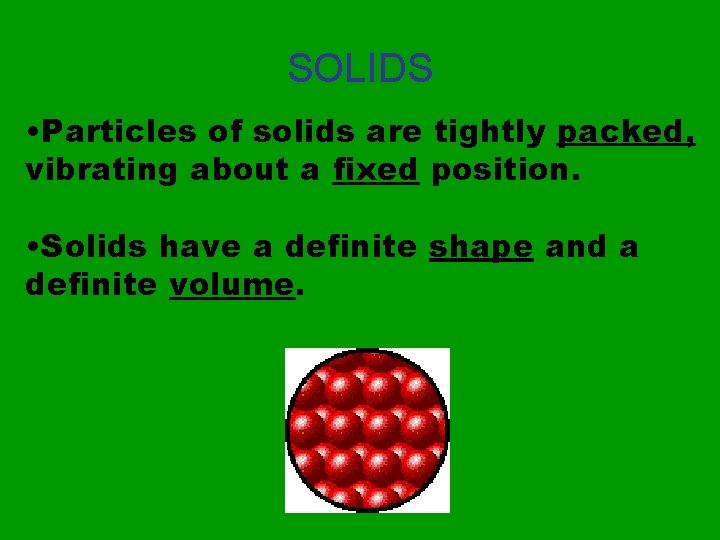
SOLIDS • Particles of solids are tightly packed, vibrating about a fixed position. • Solids have a definite shape and a definite volume.

LIQUID § Particles of liquids are tightly packed, but are far enough apart to slide over one another. § Liquids have an indefinite shape and a definite volume.

GAS § Particles of gases are very far apart and move freely. § Gases have an indefinite shape and an indefinite volume.

• http: //www. rockingham. k 12. va. us/resource s/elementary/files/2 solidliquidgas. jpg


But what happens if you raise the temperature to super-high levels… between 1000°C and 1, 000, 000°C ? Will everything just be a gas?

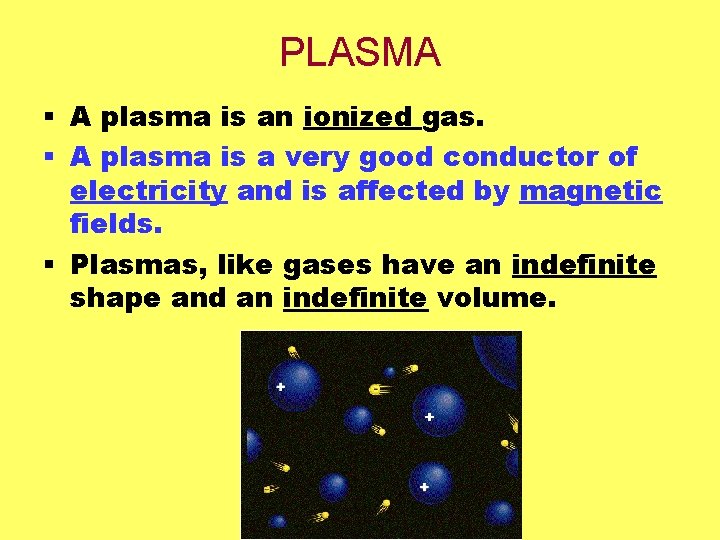
PLASMA § A plasma is an ionized gas. § A plasma is a very good conductor of electricity and is affected by magnetic fields. § Plasmas, like gases have an indefinite shape and an indefinite volume.

STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

Some places where plasmas are found… 1. Flames

2. Lightning

3. Aurora (Northern Lights)

The Sun is an example of a star in its plasma state


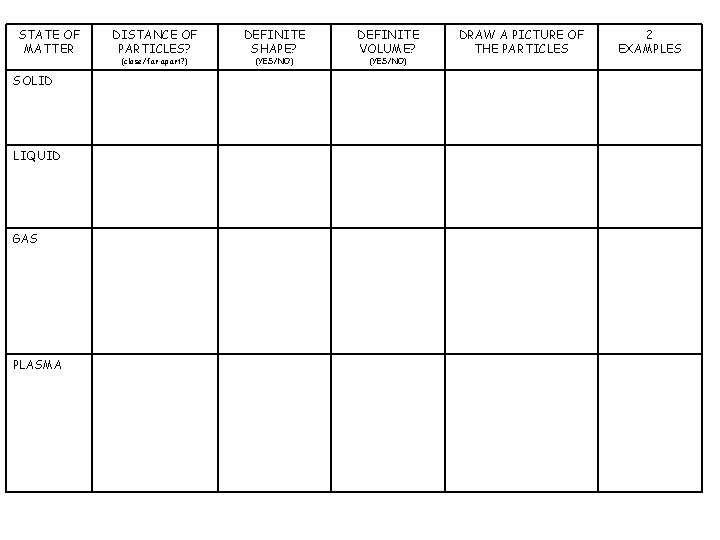
STATE OF MATTER SOLID LIQUID GAS PLASMA DISTANCE OF PARTICLES? (close/far apart? ) DEFINITE SHAPE? (YES/NO) DEFINITE VOLUME? (YES/NO) DRAW A PICTURE OF THE PARTICLES 2 EXAMPLES

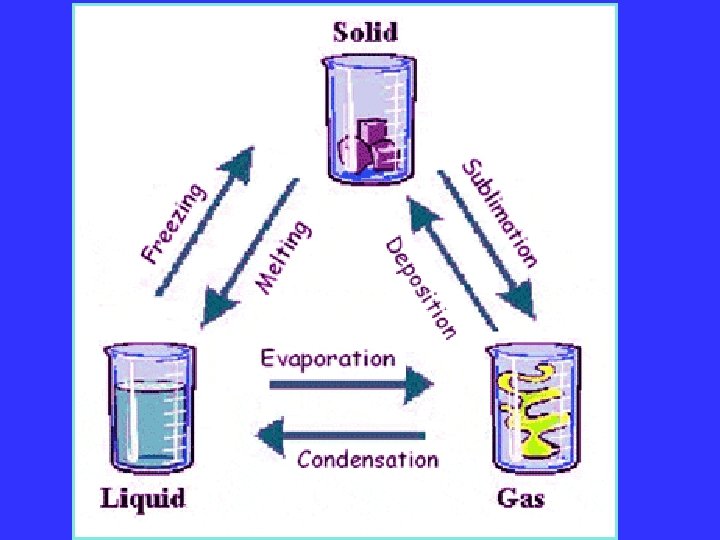
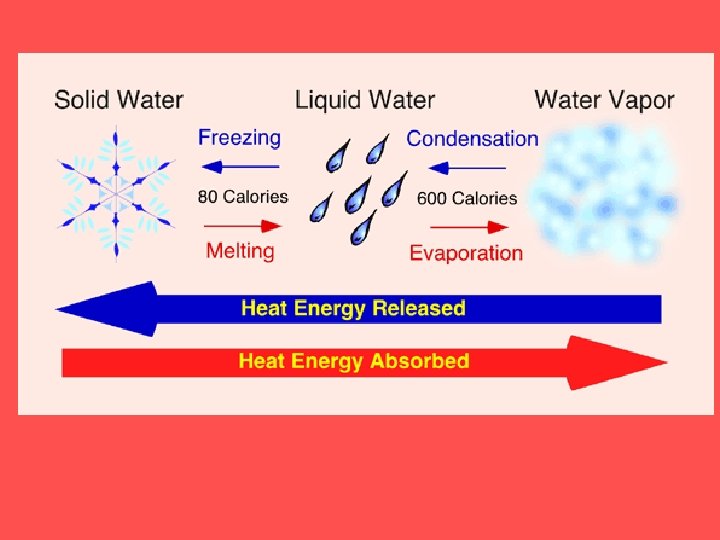
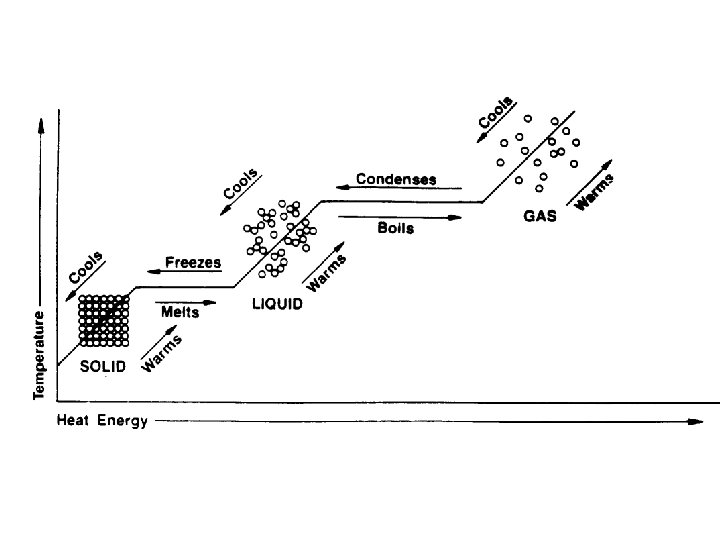
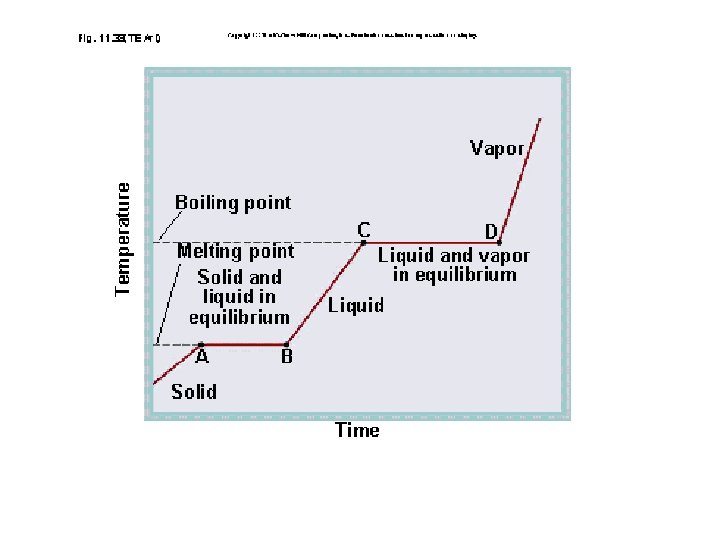
A change of state is when a substance changes from one physical form to another. This happens when heat energy is added or taken away from the matter Phase changes are a physical change


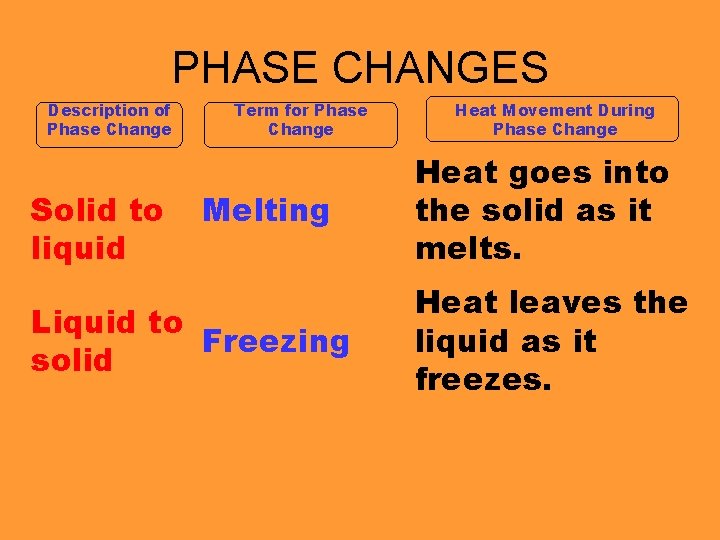
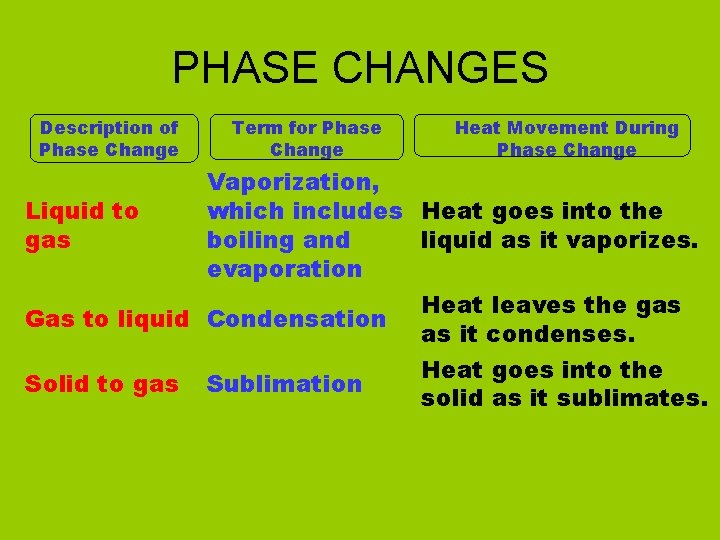
PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

PHASE CHANGES Description of Phase Change Liquid to gas Term for Phase Change Vaporization, which includes Heat goes into the boiling and liquid as it vaporizes. evaporation Gas to liquid Condensation Solid to gas Heat Movement During Phase Change Sublimation Heat leaves the gas as it condenses. Heat goes into the solid as it sublimates.



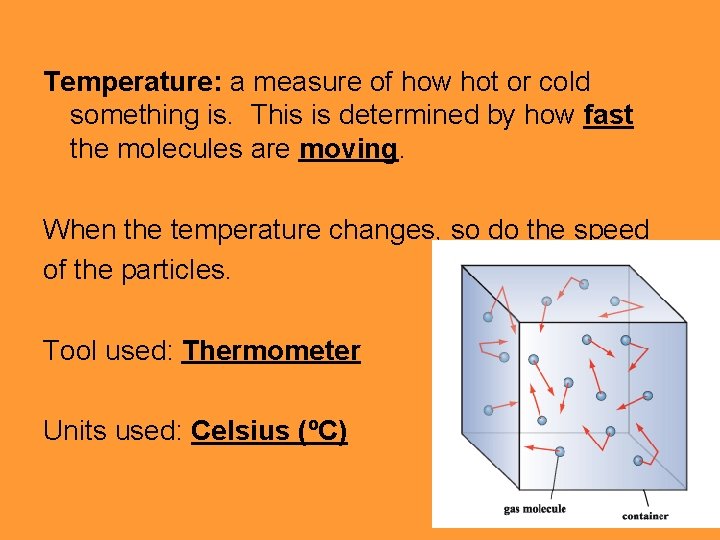
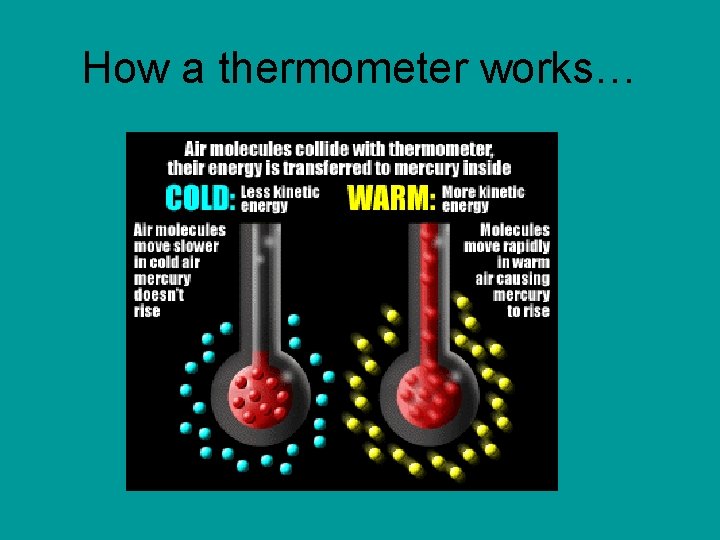
Temperature: a measure of how hot or cold something is. This is determined by how fast the molecules are moving. When the temperature changes, so do the speed of the particles. Tool used: Thermometer Units used: Celsius (ºC)

How a thermometer works…

A funny thing happens during a phase change! The temperature will change when the change of state is complete. This is because the energy is being used to break the attraction of the particles to one another.

