Matter Energy and Life Matter Forms Structure and
























- Slides: 38

Matter, Energy, and Life

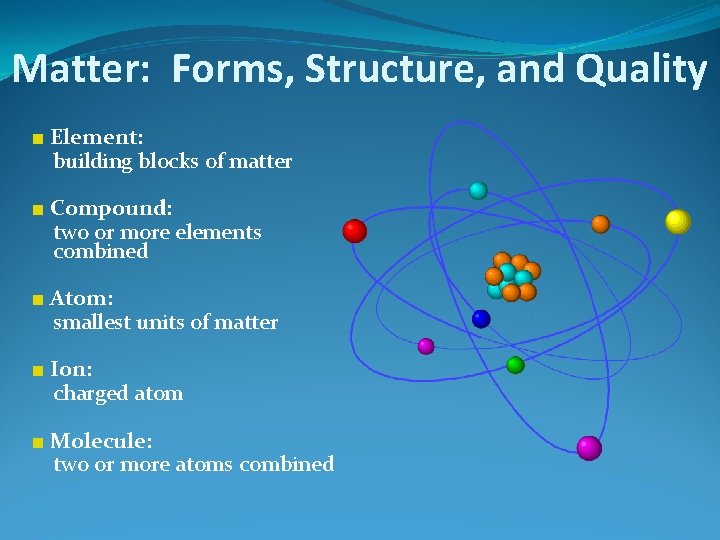
Matter: Forms, Structure, and Quality ■ Element: building blocks of matter ■ Compound: two or more elements combined ■ Atom: smallest units of matter ■ Ion: charged atom ■ Molecule: two or more atoms combined

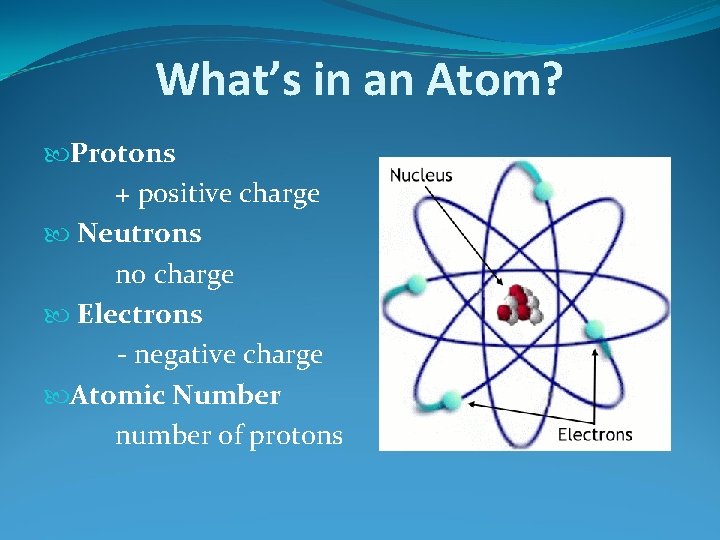
What’s in an Atom? Protons + positive charge Neutrons no charge Electrons - negative charge Atomic Number number of protons

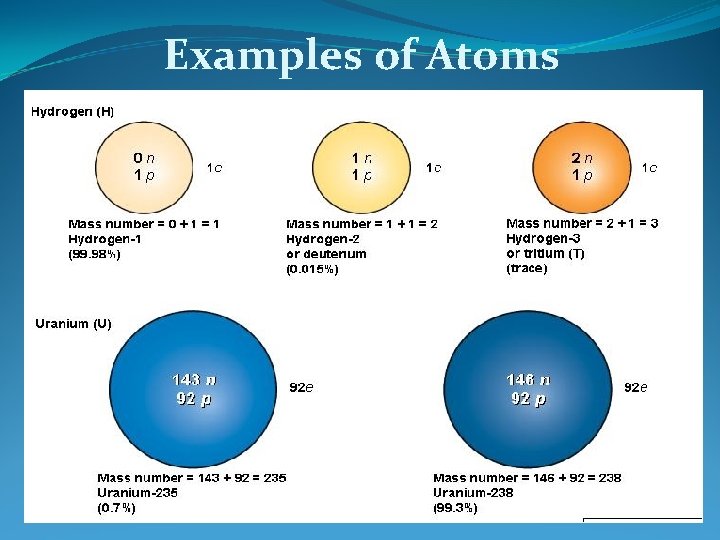
Examples of Atoms Fig. 3 -4 p. 48

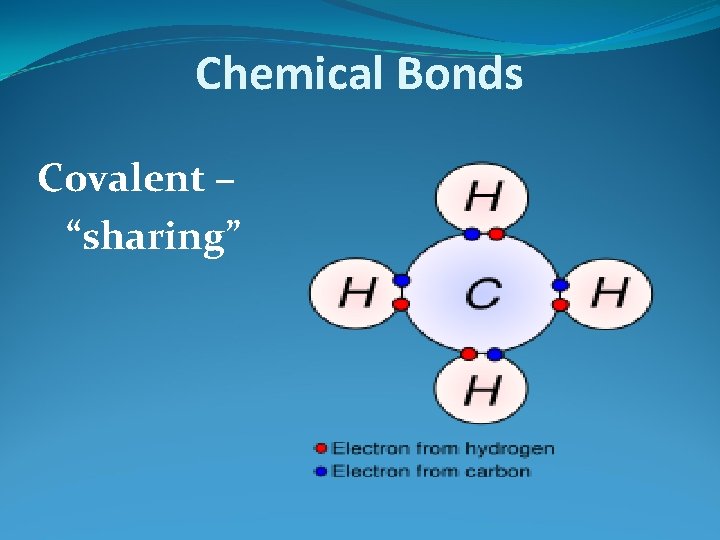
Chemical Bonds Covalent – “sharing”

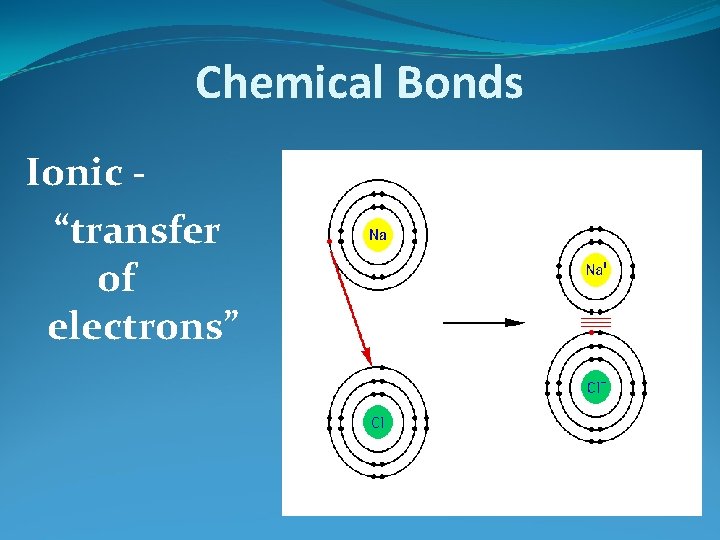
Chemical Bonds Ionic “transfer of electrons”

Organic Compounds ØLipids – fats and oils; horomones ØNucleic Acids – genetic information ØCarbohydrates – glucose, sucrose, fructose, galactose Ø Proteins – amino acids with carbon backbones; enzymes

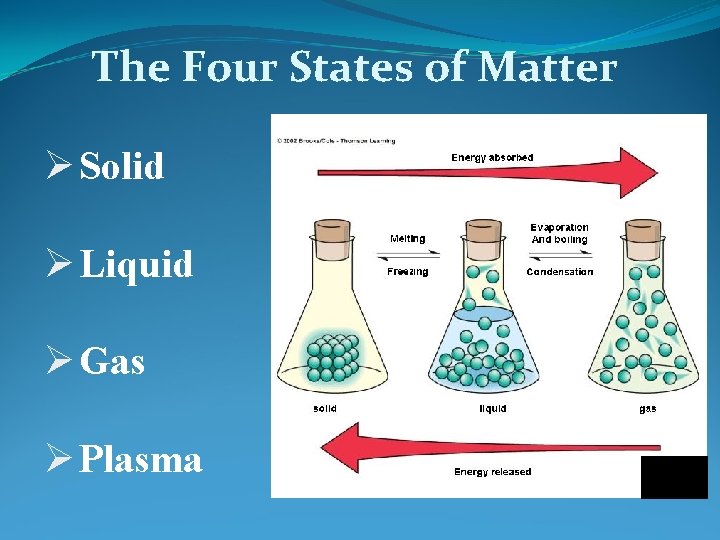
The Four States of Matter Ø Solid Ø Liquid Ø Gas Ø Plasma

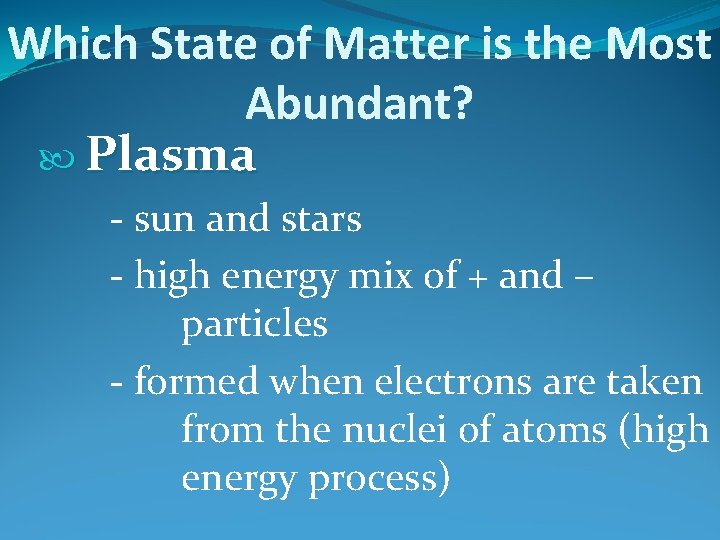
Which State of Matter is the Most Abundant? Plasma - sun and stars - high energy mix of + and – particles - formed when electrons are taken from the nuclei of atoms (high energy process)

Why is There No “Away”? Law of Conservation of Matter We cannot destroy atoms. We can only rearrange them into different spatial patterns (physical) or into different combinations (chemical). Everything we think we have “thrown away” is still here in one form or another.

Example DDT - banned, but still residues in imported coffee, tea, fruit, and other foods. - or as fallout from air masses moved long distances by wind. Law of Conservation of Matter - means we will always face the problem of what to do with wastes and pollutants.

Pollution 3 Factors that Determine the Severity of a Pollutant’s Chemical Effects: 1. Chemical Nature 2. Concentration - parts per million (ppm) 3. Persistence - measure of how long the pollutant stays in the air, water, soil, or body. Classification of Pollutants: 1. Degradable (reduced to acceptable levels) 2. Slowly Degradable (decades or longer-DDT) 3. Nondegradable (natural processes cannot break down lead, arsenic)

Energy - Ability to do work!! Work – application of force over distance (joules) Power – rate of energy flow of the rate of work done; watt = 1 joule/sec 100 watt light bulb for 10 hours = 1, 000 watt-hours or 1 k. Wh)

Forms of Energy Kinetic - energy in motion Examples: Wind, Flowing Streams, Electricity Potential - stored energy Examples: Unlit Stick of Dynamite, Rock in Hand

Forms of Energy The five main forms of energy are: Heat Chemical Electromagnetic Nuclear Mechanical

Laws of Thermodynamics FIRST LAW OF THERMODYNAMICS In all physical and chemical changes, energy is neither created nor destroyed, but it may be converted from one form to another. Energy input always equal energy output. You cannot get something for nothing in terms of energy quantity. SECOND LAW OF THERMODYNAMICS When energy is changed from one form to another, some of the useful energy is always degraded to lower quality, more dispersed, less useful energy, usually heat.

Energy Conversions In an automobile engine, fuel is burned to convert chemical energy into heat energy. The heat energy is then changed into mechanical energy.

Chemical Heat Mechanical

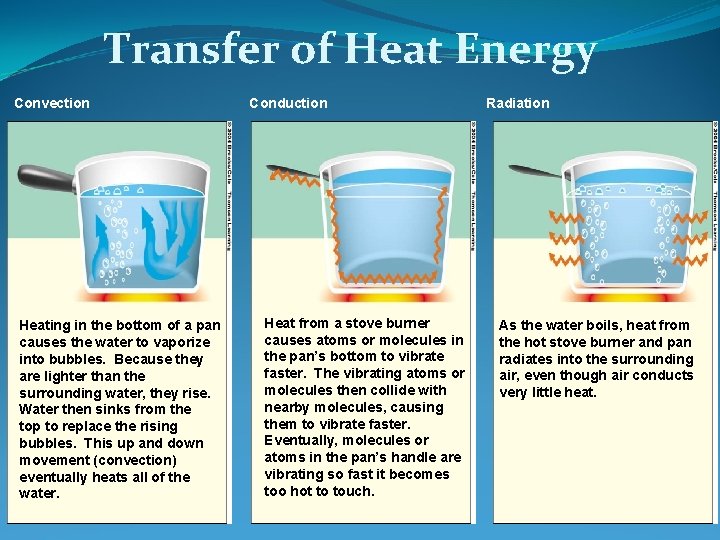
Transfer of Heat Energy Convection Heating in the bottom of a pan causes the water to vaporize into bubbles. Because they are lighter than the surrounding water, they rise. Water then sinks from the top to replace the rising bubbles. This up and down movement (convection) eventually heats all of the water. Conduction Heat from a stove burner causes atoms or molecules in the pan’s bottom to vibrate faster. The vibrating atoms or molecules then collide with nearby molecules, causing them to vibrate faster. Eventually, molecules or atoms in the pan’s handle are vibrating so fast it becomes too hot to touch. Radiation As the water boils, heat from the hot stove burner and pan radiates into the surrounding air, even though air conducts very little heat.

Energy Quality - Energy source’s ability to do useful work. High Quality Energy 1. Concentrated 2. Provides useful work Examples: Electricity, Concentrated Sunlight

Energy Continued Low Quality Energy 1. Dispersed 2. Little useful work Example: Heat dispersed in the Atlantic Ocean.

Nuclear Changes Matter undergoes a nuclear change: 1. natural radioactive decay 2. nuclear fission 3. nuclear fusion

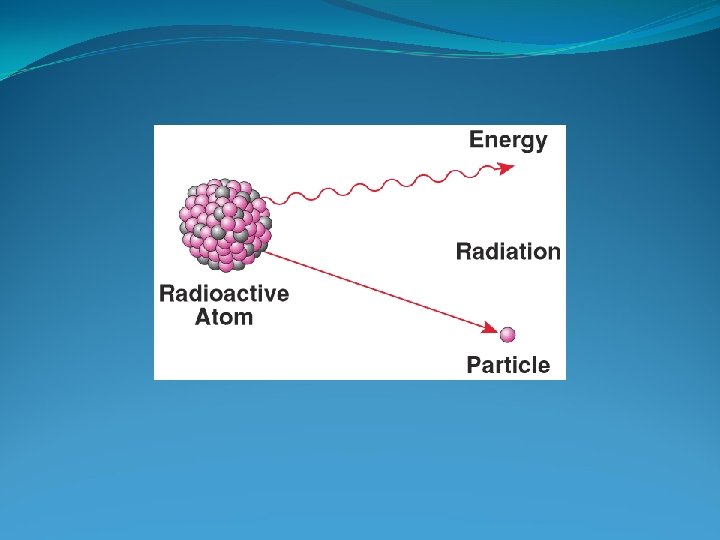
Natural Radioactive Decay A nuclear change in which unstable isotopes spontaneously emit fastmoving particles (matter), high-energy radiation, or both at a fixed rate. Unstable Isotopes are called “radioactive isotopes” - radioactive decay continues until isotope becomes stable. Isotopes have a different number of neutrons but the same number of protons.

Natural Radioactive Decay Continued Radiation emitted by radioisotopes is damaging ionizing radiation. Gamma Rays – a form of high-energy electromagnetic radiation emitted from radioisotopes. You do not want to be exposed to these waves. Alpha/Beta Particles – high-speed ionizing particles emitted from the nuclei of radioisotopes.


What is Half-Life? The amount of time needed for one-half of the nuclei in a given quantity of a radioisotope to decay and emit their radiation to form a different isotope. Decay continues, often producing a series of different radioisotopes, until a stable, nonradioactive isotope is formed. The half-life estimates how long a sample of radioactive isotope must be stored in a safe container before it decays to a safe level and can be released into the

Half-Life Continued A general rule is that such decay to a safe level takes about 10 half-lives. Example: Plutonium-239 has a half-life 24, 000 years. It is produced in nuclear reactors and used in nuclear weapon production. It must be stored safely for 240, 000 years (10 x 24, 000). Plutonium-239 can cause lung cancer when its particles are inhaled in minute amounts. Ionizing radiation exposure from alpha particles, beta particles, and gamma rays can damage cells by genetic damage (mutations of DNA) or somatic damage (tissue damage).

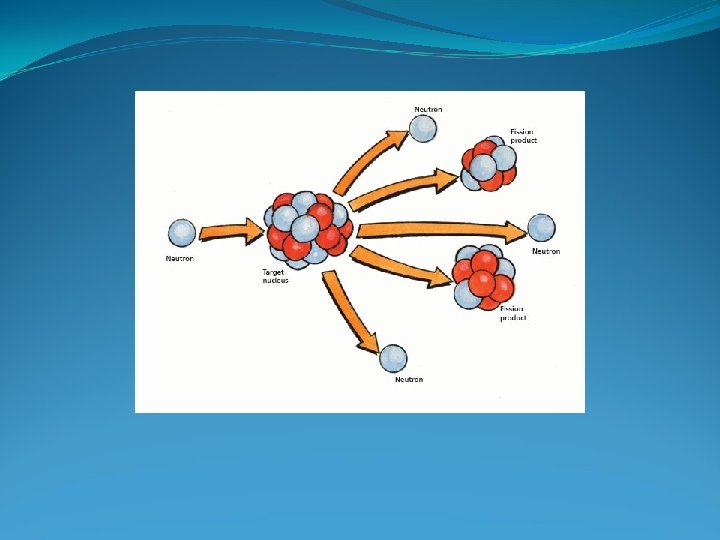
Nuclear Fission Neutrons can split apart the nuclei of certain isotopes with large mass numbers and release a large amount of energy. 1. Neutron hits the nucleus of an isotope. 2. Nucleus splits and releases 2 or 3 more neutrons and ENERGY. 3. Each of these neutrons can go on to cause additional fission. Multiple fissions create a chain reaction which releases an ENORMOUS AMOUNT OF ENERGY.

Examples of Nuclear Fission Atomic Bomb – An enormous amount of energy is released in a fraction of a second in an uncontrolled nuclear fission chain reaction. Nuclear Power Plant – The rate at which the nuclear fission chain reaction takes place is controlled. In conventional nuclear fission reactors, the splitting of uranium 235 nuclei releases energy in form of heat, which produces high-pressure steam to spin turbines and thus generate electricity.



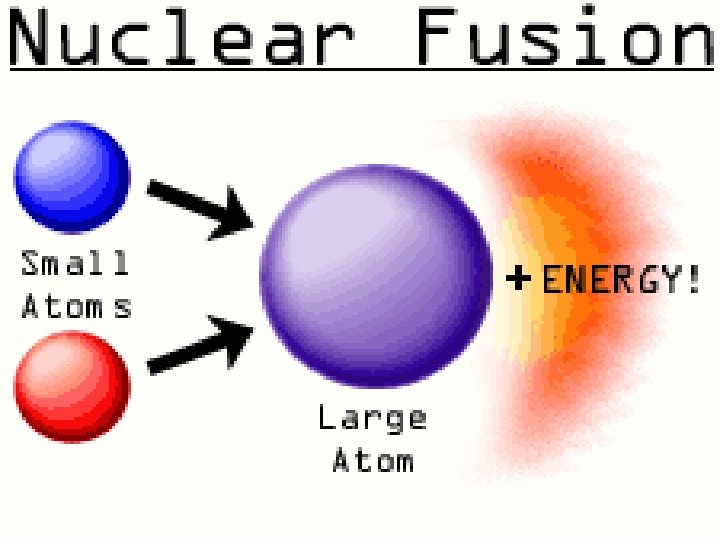
Nuclear Fusion Nuclear fusion is a nuclear change in which extremely high temperatures force the nuclei of isotopes of some lightweight atoms to fuse together and form a heavier nucleus which in turn releases large amounts of energy. Extremely high temperatures (at least 100 million o. C) are needed to force the positively charged nuclei (protons strongly repel one another) to fuse. Source of energy in sun and stars.


Sun-hydrogen isotopes fuse to make helium-energy and heat.

What are Nuclear Reactions used for? Energy Production: nuclear power plants generate electricity for our homes. Medical Technology: cancer treatment, X-rays. Nuclear Weapons: atomic bomb, hydrogen bomb.

Nuclear Power Plant

Atomic Bomb
