Matter does it matter Yesit does Chemistry is































- Slides: 56

Matter, does it matter? Yes…it does! Chemistry is the study of matter and the changes it undergoes.

Matter We define matter by using two criteria. Something is considered to be matter if it: Ø Ø Ø Has mass Takes up space What are some examples?

Matter

Not Matter Energy Light Ideas Happiness Love

Classifying matter - Why? 1. To organize what we have 2. When things are organized – we know what we have 3. And more importantly, we know what we do NOT have.

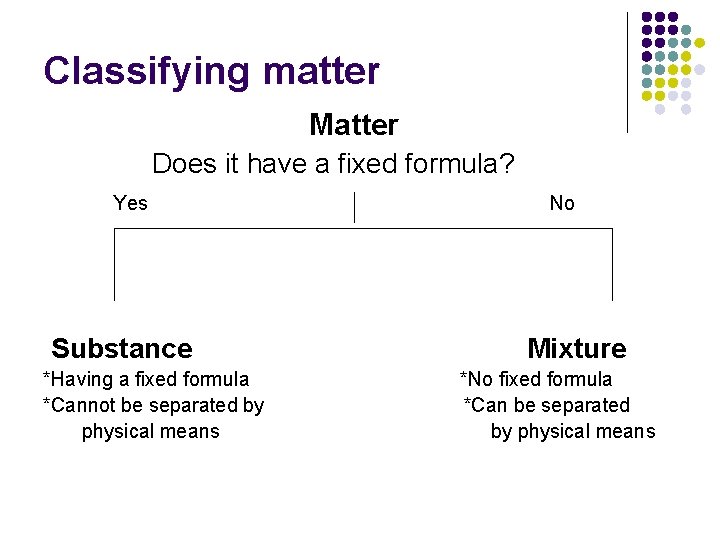
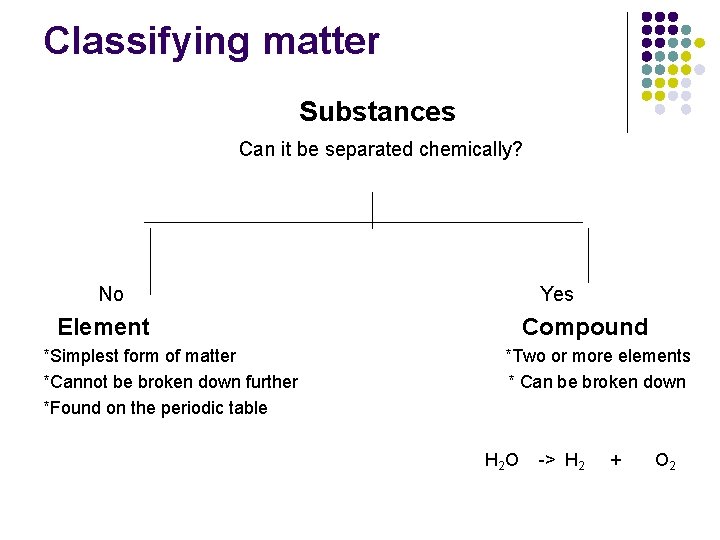
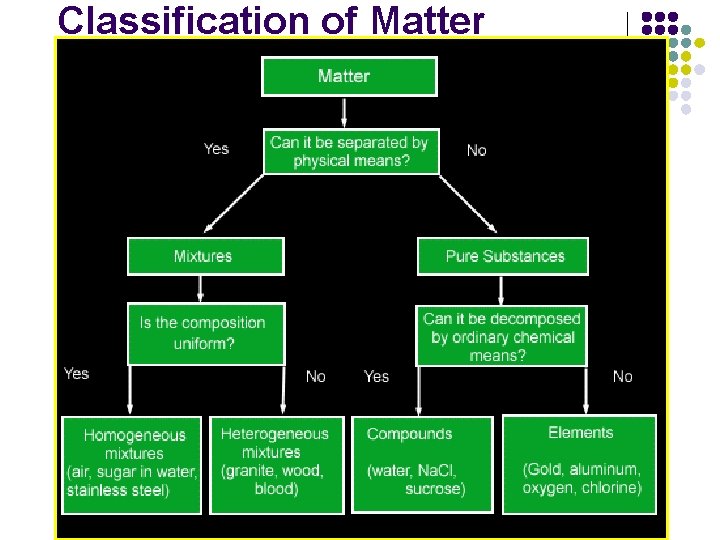
Classifying matter Matter Does it have a fixed formula? Yes Substance *Having a fixed formula *Cannot be separated by physical means No Mixture *No fixed formula *Can be separated by physical means

Classifying matter Substances Can it be separated chemically? No Yes Element *Simplest form of matter *Cannot be broken down further *Found on the periodic table Compound *Two or more elements * Can be broken down H 2 O -> H 2 + O 2

Substances: element or compound l l Elements- simplest kind of matter l cannot be broken down any simpler and still have properties of that element! l all one kind of atom. Compounds are substances that can be broken down only by chemical methods l when broken down, the pieces have completely different properties than the original compound. l made of two or more atoms, chemically combined (not just a physical blend!)

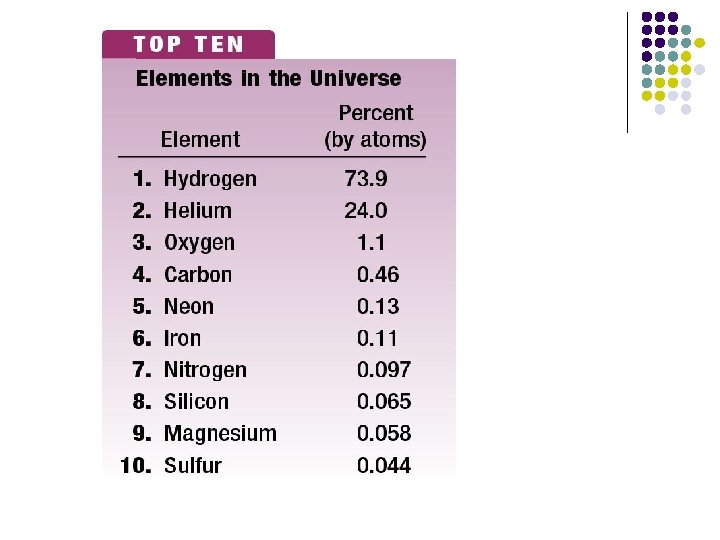
Symbols & Formulas l Currently, there are 117 elements -Only 92 of the 115 presently known elements occur naturally. l l Elements have a 1 or two letter symbol, and compounds have a formula. An element’s first letter always capitalized; if there is a second letter, it is written lowercase: B, Ba, C, Ca, H, He



Some elements pair up and only exist as diatomic molecules Hydrogen, Nitrogen, Fluorine, Oxygen, Iodine, Chlorine, and Bromine are always found as diatomic molecules: How do I remember this? H N F O I Cl Br • HONCl. FIBr (say HONKLE-fibber) • Br. INCl. HOF (say Brinckle-hoff) • I Have No Bright Or Clever Friends • Cl. IF H Bron • HOFBr. INCl Twins (twins because they exist in pairs) • There are seven such elements. The first one is the first element Hydrogen; the rest form a 7 on the periodic table: N, O, F across, then going down Cl, Br, I.

Elements vs. Compounds l Compounds can be broken down into simpler substances by chemical means, but elements cannot. l A “chemical change” is a change that produces matter with a different composition than the original matter.

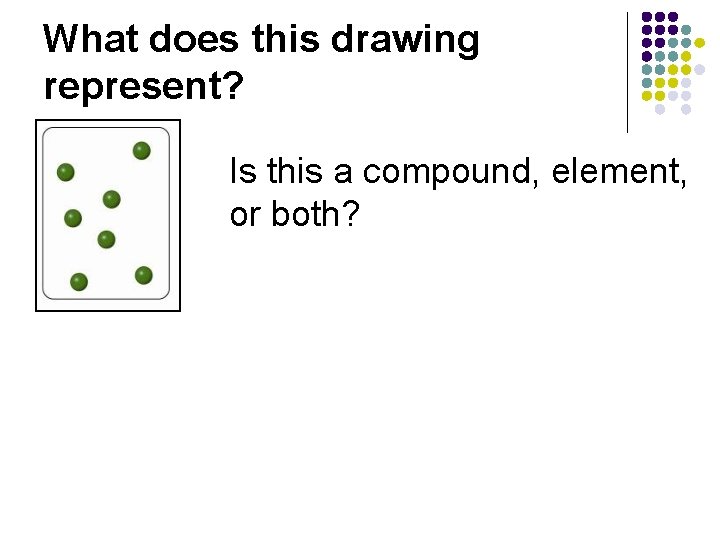
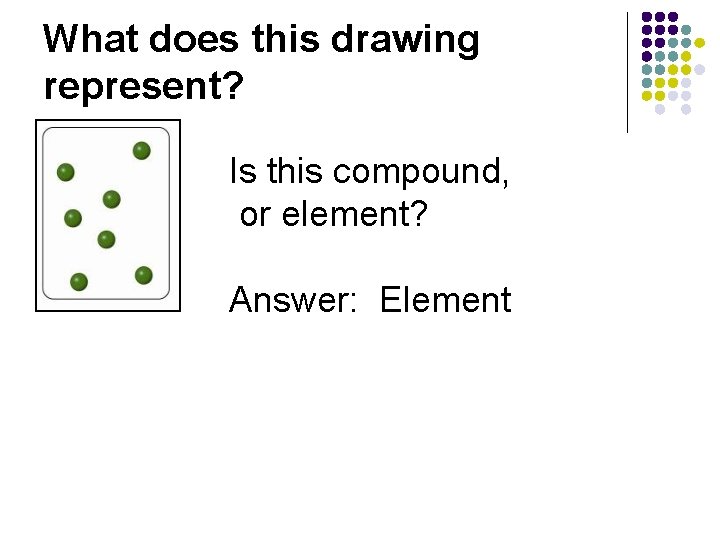
What does this drawing represent? Is this a compound, element, or both?

What does this drawing represent? Is this compound, or element? Answer: Element

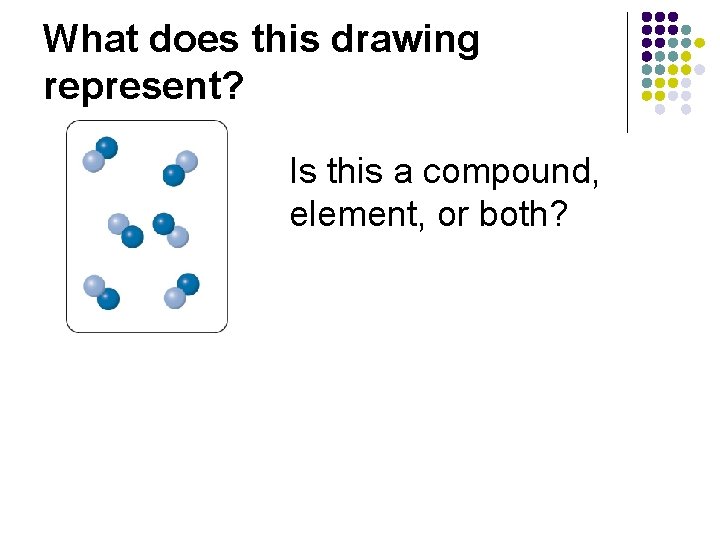
What does this drawing represent? Is this a compound, element, or both?

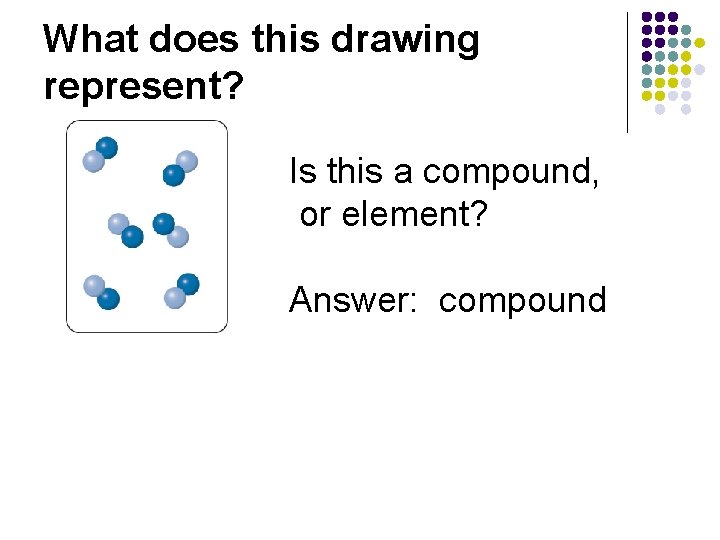
What does this drawing represent? Is this a compound, or element? Answer: compound

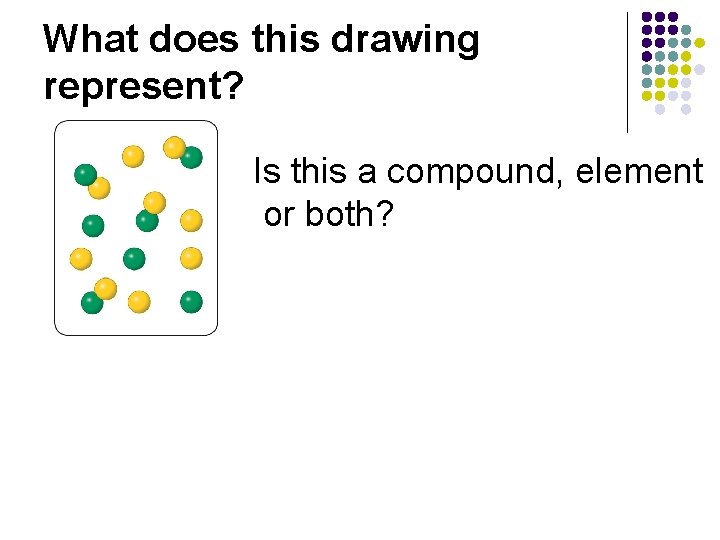
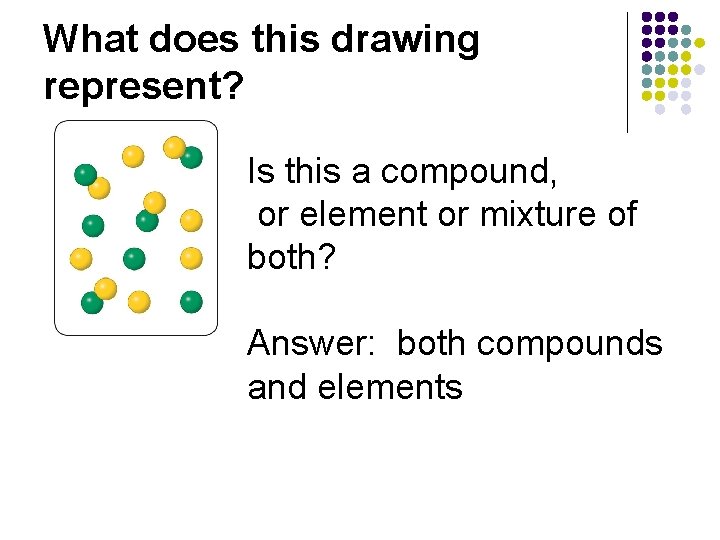
What does this drawing represent? Is this a compound, element or both?

What does this drawing represent? Is this a compound, or element or mixture of both? Answer: both compounds and elements

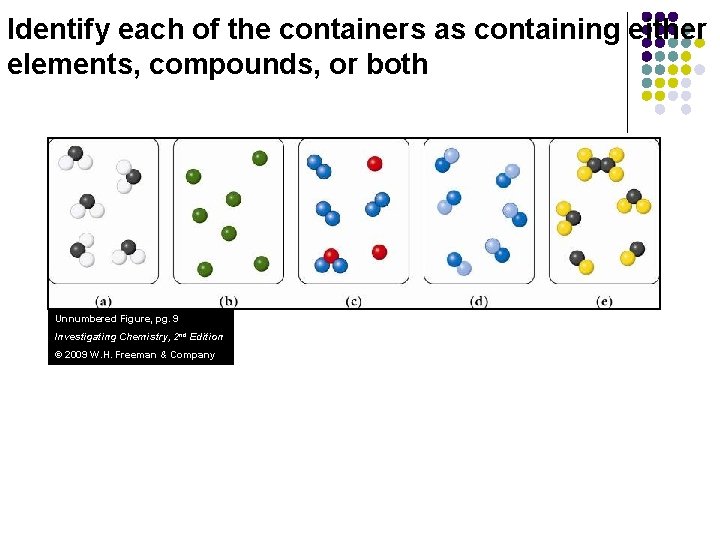
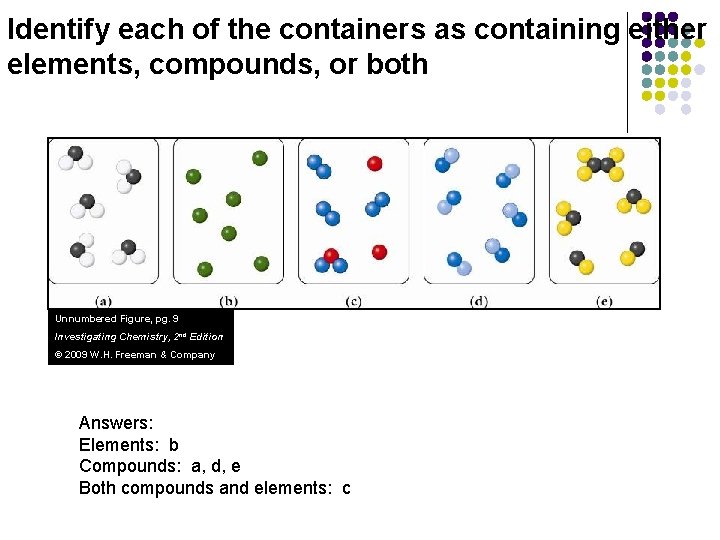
Identify each of the containers as containing either elements, compounds, or both Unnumbered Figure, pg. 9 Investigating Chemistry, 2 nd Edition © 2009 W. H. Freeman & Company

Identify each of the containers as containing either elements, compounds, or both Unnumbered Figure, pg. 9 Investigating Chemistry, 2 nd Edition © 2009 W. H. Freeman & Company Answers: Elements: b Compounds: a, d, e Both compounds and elements: c

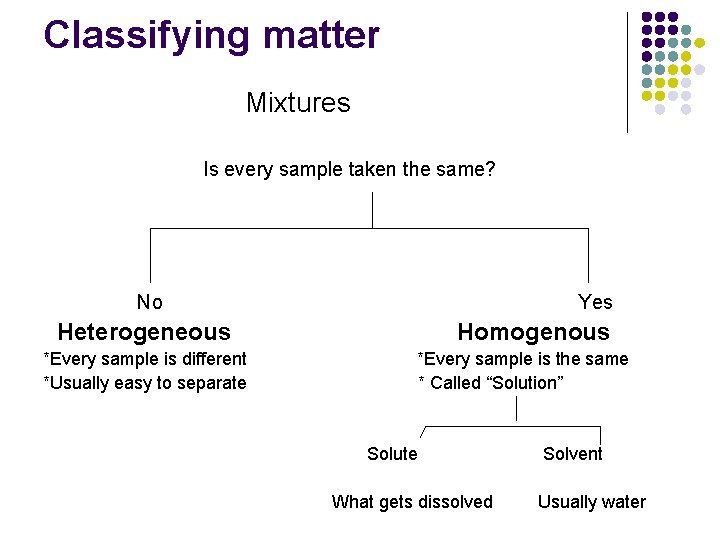
Classifying matter Mixtures Is every sample taken the same? No Yes Heterogeneous *Every sample is different *Usually easy to separate Homogenous *Every sample is the same * Called “Solution” Solute What gets dissolved Solvent Usually water

Mixtures l Have variable composition. Homogeneous Mixture § Having visibly indistinguishable parts; solution. Heterogeneous Mixture § Having visibly distinguishable parts. Copyright © Cengage Learning. All rights reserved

Homogeneous Mixtures Copyright © Cengage Learning. All rights reserved

Homogeneous vs. Heterogeneous Mixtures Copyright © Cengage Learning. All rights reserved

Classification of Matter

Describing Matter l Properties used to describe matter can be classified as: 1) Extensive – depends on the amount of matter in the sample - Mass, volume, calories are examples 2) Intensive – depends on the type of matter, not the amount present - Hardness, Density, Boiling Point

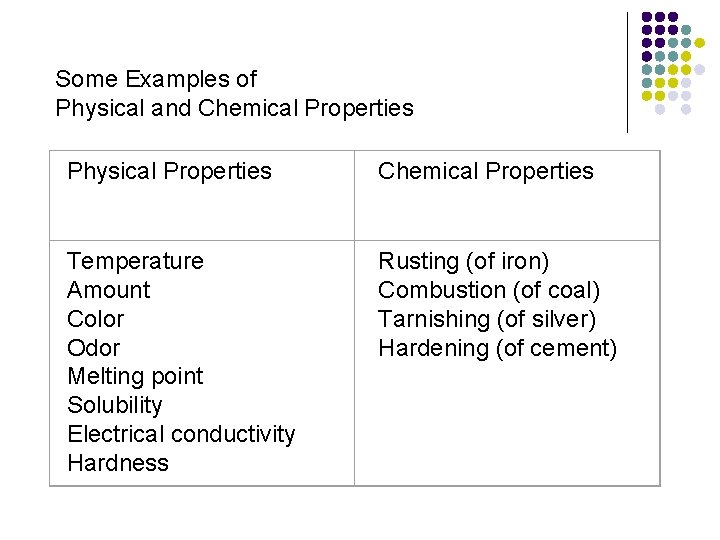
Properties are… Words that describe matter (adjectives) l Physical Properties- a property that can be observed and measured without changing the material’s composition. l Examples- color, hardness, m. p. , b. p. l Chemical Properties- a property that can only be observed by changing the composition of the material. l Examples- ability to burn, decompose, ferment, react with, etc.

Some Examples of Physical and Chemical Properties Physical Properties Chemical Properties Temperature Amount Color Odor Melting point Solubility Electrical conductivity Hardness Rusting (of iron) Combustion (of coal) Tarnishing (of silver) Hardening (of cement)

Properties of Compounds Quite different properties than their component elements. l Due to a CHEMICAL CHANGE, the resulting compound has new and different properties: • Table sugar – carbon, hydrogen, oxygen • Sodium chloride – sodium, chlorine • Water – hydrogen, oxygen l

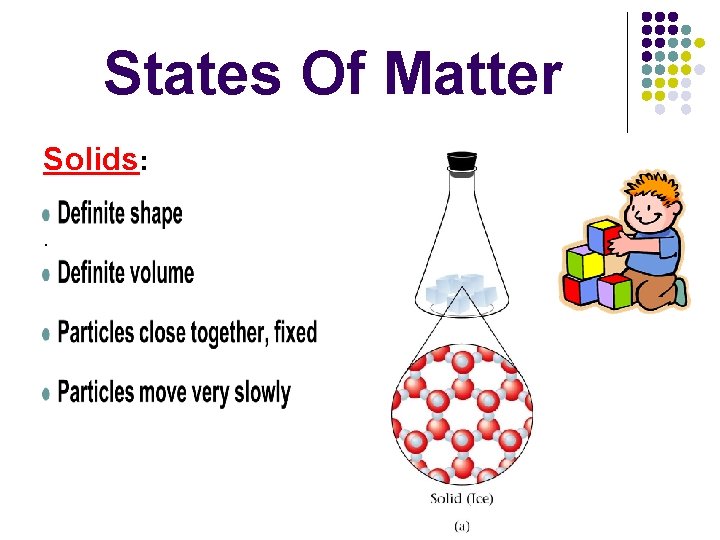
States Of Matter Solids: .

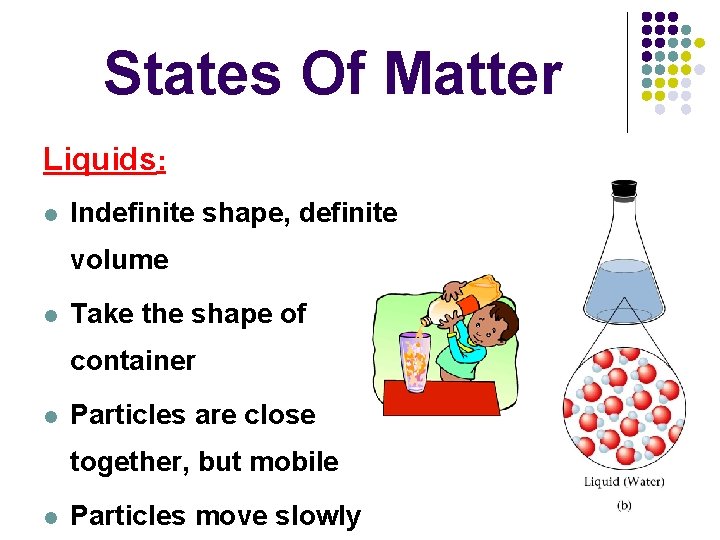
States Of Matter Liquids: l Indefinite shape, definite volume l Take the shape of container l Particles are close together, but mobile l Particles move slowly

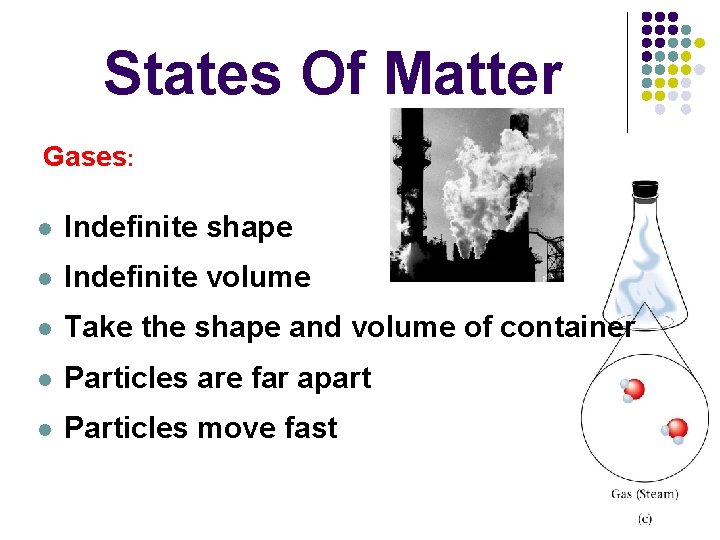
States Of Matter Gases: l Indefinite shape l Indefinite volume l Take the shape and volume of container l Particles are far apart l Particles move fast

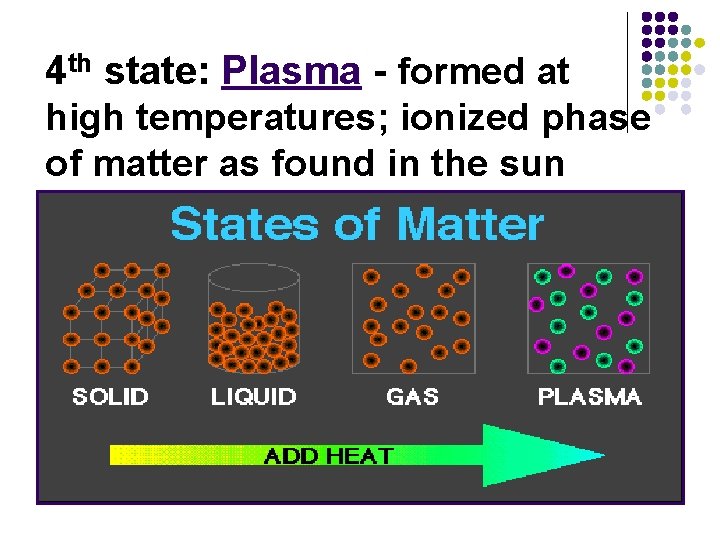
States Of Matter Plasma: Energized gases l Do not have a fixed volume. They are mostly empty space and can be compressed. l Do not have a fixed shape. They tend to fill the entire container.

States Of Matter How do we change states of matter? It requires energy. What does the energy do? It makes the molecules move. This causes friction, which results in heat being generated

4 th state: Plasma - formed at high temperatures; ionized phase of matter as found in the sun

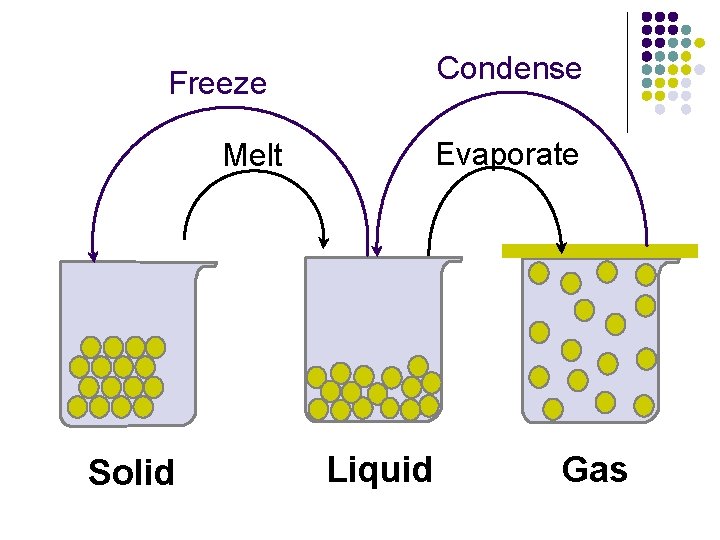
Condense Freeze Evaporate Melt Solid Liquid Gas

Learning Check S 1 Match: (1) solid, (2) liquid, or (3) gas. ____ A. Has a definite volume, but shape of the container. ____ B. Its particles are moving rapidly. ____ C. Fills the volume of a container. ____ D. Particles are in a fixed structure. ____ E. Particles are close together, but mobile. 38

Solution S 1 Match: (1) solid, (2) liquid, or (3) gas. _2_ A. Has a definite volume, but shape of the container. _3_ B. Its particles are moving rapidly. _3_ C. Fills the volume of a container. _1_ D. Particles are in a fixed structure. _2_ E. Particles are close together, but mobile. 39

Physical vs Chemical Properties are - Words that describe matter (adjectives) l Physical Properties- a property that can be observed and measured without changing the material’s composition. l Examples- color, hardness, m. p. , b. p. l Chemical Properties- a property that can only be observed by changing the composition of the material. l Examples- ability to burn, decompose, ferment, react with, etc.

Physical vs. Chemical Change Physical change will change the visible appearance, without changing the composition of the material. l Boil, melt, cut, bend, split, crack l Is boiled water still water? Ø Can be reversible, or irreversible l Chemical change - a change where a new form of matter is formed. l Rust, burn, decompose, ferment l

Physical and Chemical Changes How to tell the difference

Physical Change in the form of a substance, not in its chemical composition. § l Example: boiling or freezing water Can be used to separate a mixture into pure compounds, but it will not break compounds into elements. § § § Distillation Filtration Chromatography Copyright © Cengage Learning. All rights reserved

Physical Change l. Substance may seem different, but the way the atoms link up is the same.

It’s a physical change if l. It changes shape or size l. It dissolves l. It changes phase (freezes, boils, evaporates,

Chemical Change l. Changes the way the molecules link up l. Makes new substances

It’s a chemical change if…. l. It burns l Temperature changes without heating/cooling

It’s a chemical change if. . . l It bubbles (makes a gas)

It’s a chemical change if. . . l It changes color l It forms a precipitate

What kind of change is it if someone. . . l Tears up paper? Physical change l Mixes salt and water? Physical change

What kind of change is it if someone. . . l Burns paper? Chemical change l Evaporates salt water? Physical change

What kind of change is it if someone. . . l. Mixes vinegar and baking soda? Chemical change

Chemical Change A change in which one or more substances are converted into different substances. Heat and light are often evidence of a chemical change.

Chemical Changes The ability of a substance to undergo a specific chemical change is called a chemical property. • iron plus oxygen forms rust, so the ability to rust is a chemical property of iron l During a chemical change (also called chemical reaction), the composition of matter always changes. l

Recognizing Chemical Changes Energy is absorbed or released (temperature changes hotter or colder) 2) Color changes 3) Gas production (bubbling, fizzing, or odor change; smoke) 4) formation of a precipitate - a solid that separates from solution (won’t dissolve) 5) Irreversibility - not easily reversed But, there are examples of these that are not chemical – boiling water bubbles, etc. 1)

Conservation of Mass During any chemical reaction, the mass of the products is always equal to the mass of the reactants. l All the mass can be accounted for: l Burning of wood results in products that appear to have less mass as ashes; where is the rest? l l Law of conservation of mass