Matter chapters 1 3 It really does matter


















































- Slides: 52

Matter chapters 1 -3

It really does matter! Chemystery Physicological Coup de eta Elements, compounds and mixtures oh my! 10 10 10 20 20 20 30 30 30 40 40 40 50 50 50

Question 1 - 10 • What has mass and takes up space?

Answer 1 – 10 • Matter

Question 1 - 20 • Which units would be best for describing the volume of mercury (liquid) used in an experiment

Answer 1 – 20 • milliliters

Question 1 - 30 • what is mass?

Answer 1 – 30 • The measure of how much of an object you have.

Question 1 - 40 • What happens to a solid object with a density that is less than water when it is placed in water?

Answer 1 – 40 • It floats

Question 1 - 50 • Which property of matter is a measure of the gravitational force?

Answer 1 – 50 • weight

Question 2 - 10 • An element’s ability to react with acid is an example of a?

Answer 2 – 10 • Chemical property

Question 2 - 20 • How does a physical change differ from a chemical change?

Answer 2 – 20 • In a chemical change the substance changes into another substance, a physical change will have the same substance in the end.

Question 2 - 30 • Which of the following is NOT the result of a chemical change? • a. • soured milk • c. • ground flour • b. • rusted metal • digested food

Answer 2 – 30 • Ground flour

Question 2 - 40 • • • Melting crayons is an example of a a. physical property. c. chemical property. b. physical change. d. chemical change.

Answer 2 – 40 • Physical change

Question 2 - 50 • What is reactivity?

Answer 2 – 50 • The ability to form a bond or exchange electrons

Question 3 - 10 • Boiling point, melting point, and density are some of an element’s?

Answer 3 – 10 • Physical properties

Question 3 - 20 • Why would dissolving salt in water be considered a physical change?

Answer 3 – 20 • The water and the salt are not chemically combined and can be separated out.

Question 3 - 30 • What kinds of changes in substances are always physical changes?

Answer 3 – 30 • various

Question 3 - 40 • If you poured three liquids (that do not mix completely) into a beaker, how could you tell which one is the densest liquid?

Answer 3 – 40 • It would be at the bottom.

Question 3 - 50 • Which physical property of matter describes the relationship between mass and volume?

Answer 3 – 50 • density

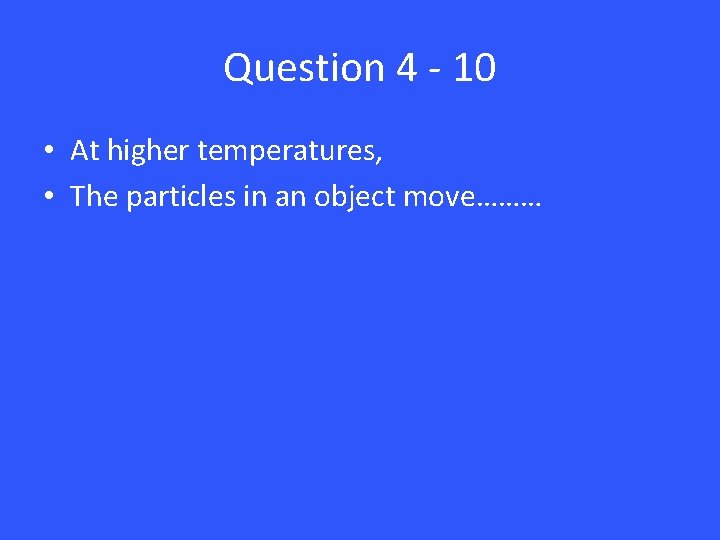
Question 4 - 10 • At higher temperatures, • The particles in an object move………

Answer 4 – 10 • Faster and farther apart

Question 4 - 20 • • • A gas a. has a definite volume but no definite shape. b. has a definite shape but no definite volume. c. has fast-moving particles. d. has particles that are always close together.

Answer 4 – 20 • Has fast moving particles

Question 4 - 30 • In a solid, the particles…. .

Answer 4 – 30 • Are vibrating and are locked close together

Question 4 - 40 • The reverse of condensation is?

Answer 4 – 40 • evaporation

Question 4 - 50 • What is the term for going directly to the gaseous state from a solid state?

Answer 4 – 50 • sublimation

Question 5 - 10 • How do elements join to form compounds?

Answer 5 – 10 • By bonding or chemical means

Question 5 - 20 • How can a compound be broken down?

Answer 5 – 20 • By breaking bonds or by chemical means

Question 5 - 30 • Most metals are?

Answer 5 – 30 • various

Question 5 - 40 • What is formed when particles of two or more substances are distributed evenly among each other?

Answer 5 – 40 • A solution

Question 5 - 50 • Why is oxygen so reactive

Answer 5 – 50 • It has electrons missing in it’s outer shell.