MATTER Chapter 4 Pure Substances and Mixtures Matter

MATTER Chapter 4

Pure Substances and Mixtures Matter can be divided into two groups: Pure substances: all the particles of a substance are the same Or Mixtures : a sample contains different types of particles
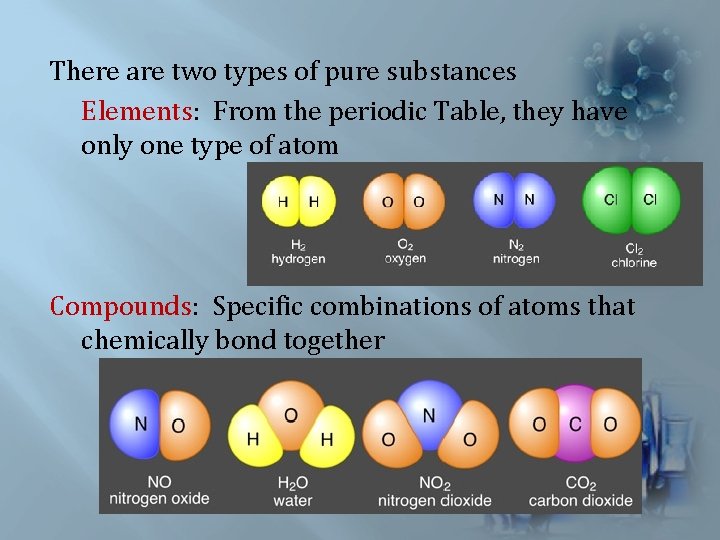
There are two types of pure substances Elements: From the periodic Table, they have only one type of atom Compounds: Specific combinations of atoms that chemically bond together
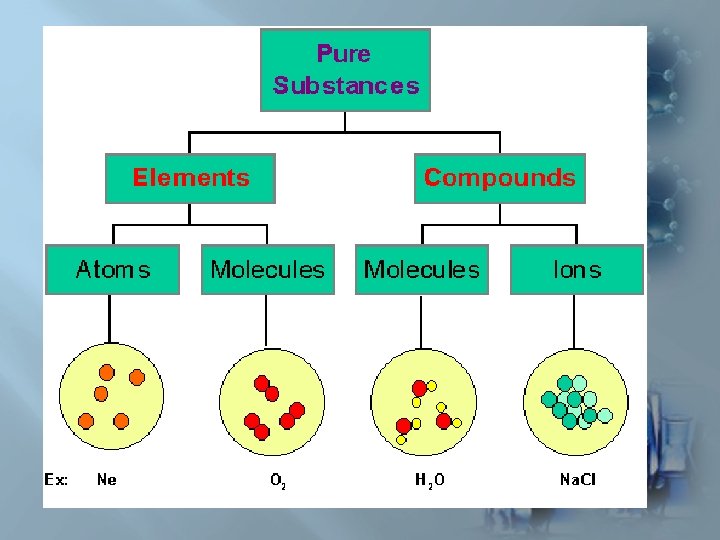

Elements: Cannot be broken down by ordinary chemical reactions Compounds: : Can be broken down by ordinary chemical reactions
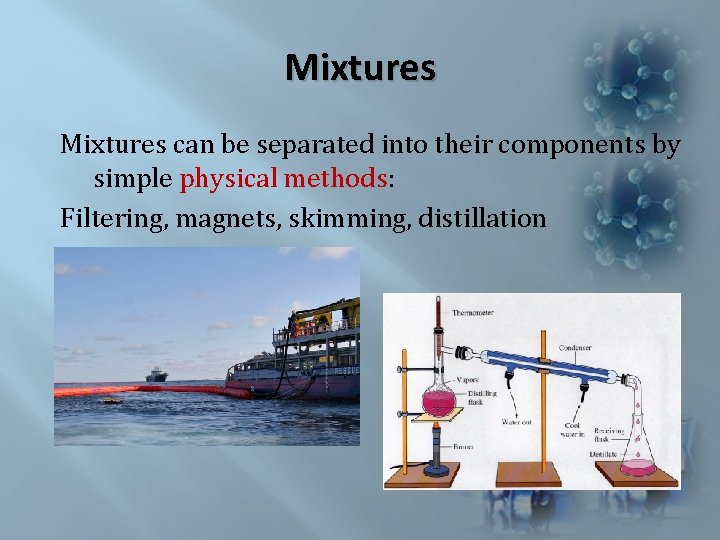
Mixtures can be separated into their components by simple physical methods: Filtering, magnets, skimming, distillation

Which is it? Mixture Element Compound
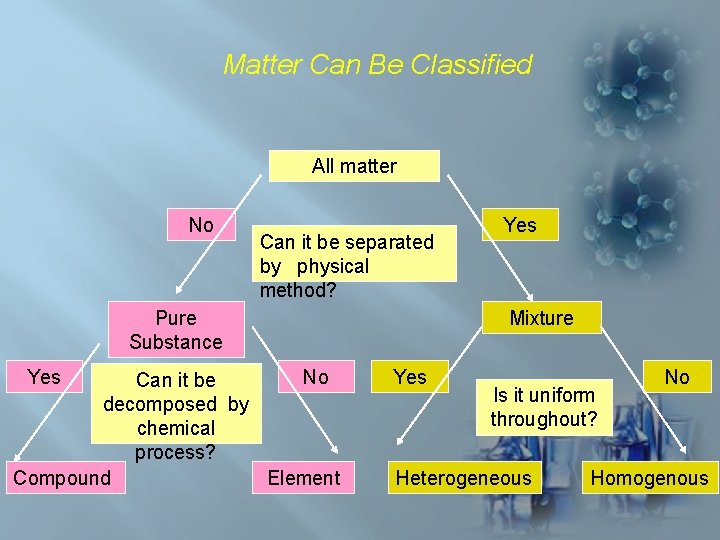
Matter Can Be Classified All matter No Can it be separated by physical method? Pure Substance Yes No Can it be decomposed by chemical process? Compound Element Yes Mixture Yes Is it uniform throughout? Heterogeneous No Homogenous

Properties of Matter Physical Property: A characteristic of a substance that can be observed and measured without changing the identity of the substance. Chemical property: The ability of a substance to react and form a new substance.

What are some Physical Properties? Properties you can see, such as color, shape, hardness, and texture, viscosity, solubility conductivity… Properties that are easily measured, such as mass, volume, density, melting point, boiling point, Density… Which properties are Quantitative; Which are qualitative? Is the ability to be attracted to a magnet a physical property?

State: Is this a physical of a chemical property?

Density is a measure of the amount of matter in a certain amount of space. Formula: Density =Mass Volume D=M V

Let’s Try Some Density Problems 1) If the mass of a rock is 500 g and its volume is 25 cm 3, what is its density? 2) If the density of a liquid is 1. 2 g/m. L, and its volume is 10 m. L, what is its mass? 3)If Bob’s mass is 80 kg and his density is 1. 6 kg/L, what is his volume?

Chemical Properties Chemical properties describe substances’ ability to change into a different substance. Reactivity with water , oxygen, acids, other substances. Combustibility Stability, toxicity
- Slides: 14